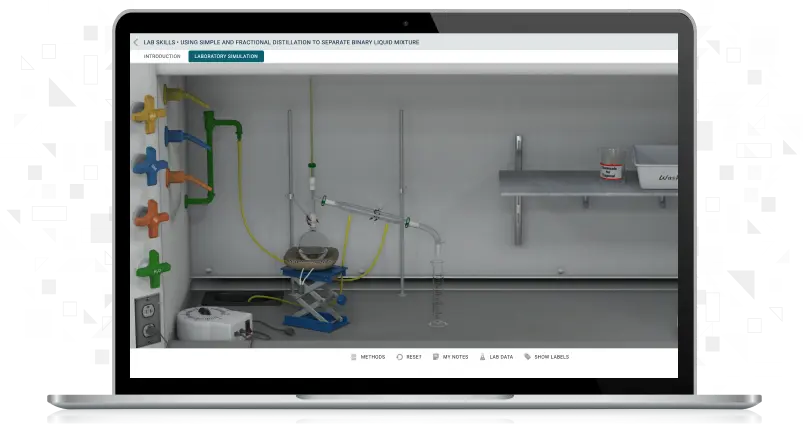
Using Simple and Fractional Distillation to Separate Binary Liquid Mixture
Students will separate an unknown mixture containing two miscible liquids and separate it into its components. Using simple distillation and fractional distillation, students use the same unknown mixture to determine each liquid component's boiling point and identity.