
C05_024A
y
y
x
x
Before collision
Electron
Speed
=
0
Photon
λ
After collision
Electron’s speed
increases.
Photon’s
wavelength
increases.
λ'
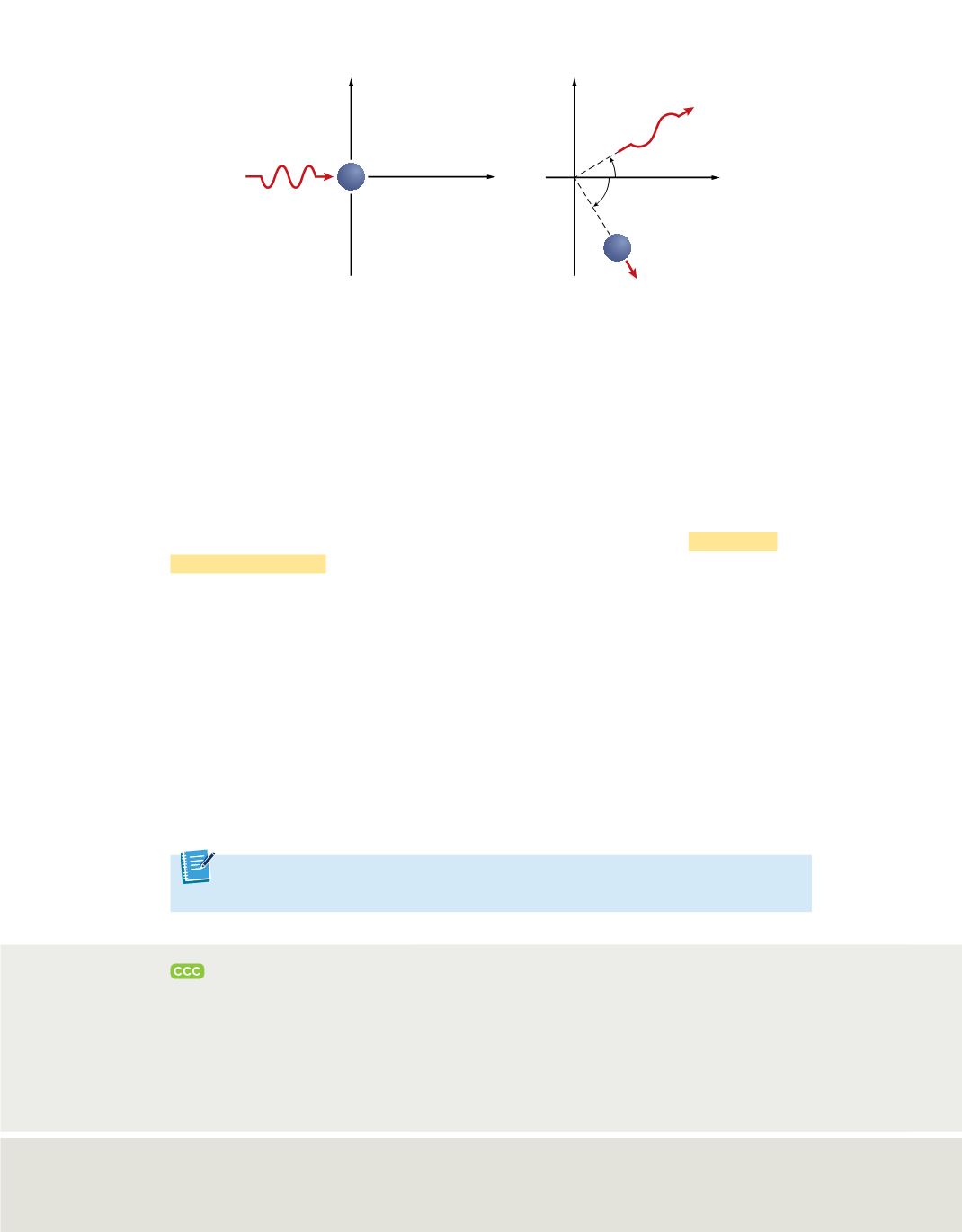
Now imagine trying to determine an electron’s location by “bumping” it with a
high-energy photon. Because such a photon has about the same energy as an electron,
the interaction between the two particles changes both the wavelength of the photon
and the position and velocity of the electron, as shown in
Figure 14
. In other words, the
act of observing the electron produces a significant, unavoidable uncertainty in the
position and motion of the electron. Heisenberg’s analysis of interactions, such as those
between photons and electrons, led him to his historic conclusion. The
Heisenberg
uncertainty principle
states that it is fundamentally impossible to know precisely both
the velocity and position of a particle at the same time.
Although scientists of the time found Heisenberg’s principle difficult to accept, it has
been proven to describe the fundamental limitations of what can be observed. The
interaction of a photon with a macroscopic object such as a helium-filled balloon has so
little effect on the balloon that the uncertainty in its position is too small to measure.
But that is not the case with an electron moving at 6
×
10
6
m/s near an atomic nucleus.
The uncertainty of the electron’s position is at least 10
-
9
m, about 10 times greater than
the diameter of the entire atom.
The Heisenberg uncertainty principle also means that it is impossible to assign fixed
paths for electrons like the circular orbits in Bohr’s model. The only quantity that can be
known is the probability for an electron to occupy a certain region around the nucleus.
Figure 14
When a photon interacts with an electron at rest, both the velocity and the position of the
electron are modified. This illustrates the Heisenberg uncertainty principle. It is impossible to know at the
same time the position and the velocity of a particle.
Explain
Why has the photon’s energy changed?
Get It?
Identify
the only quantity of an electron’s orbit that can be determined.
CROSSCUTTING CONCEPTS
Cause and Effect
What empirical evidence did scientists have that
supports the claim that electrons have both particle and wave properties?
Lesson 2 • Quantum Theory and the Atom
121









