
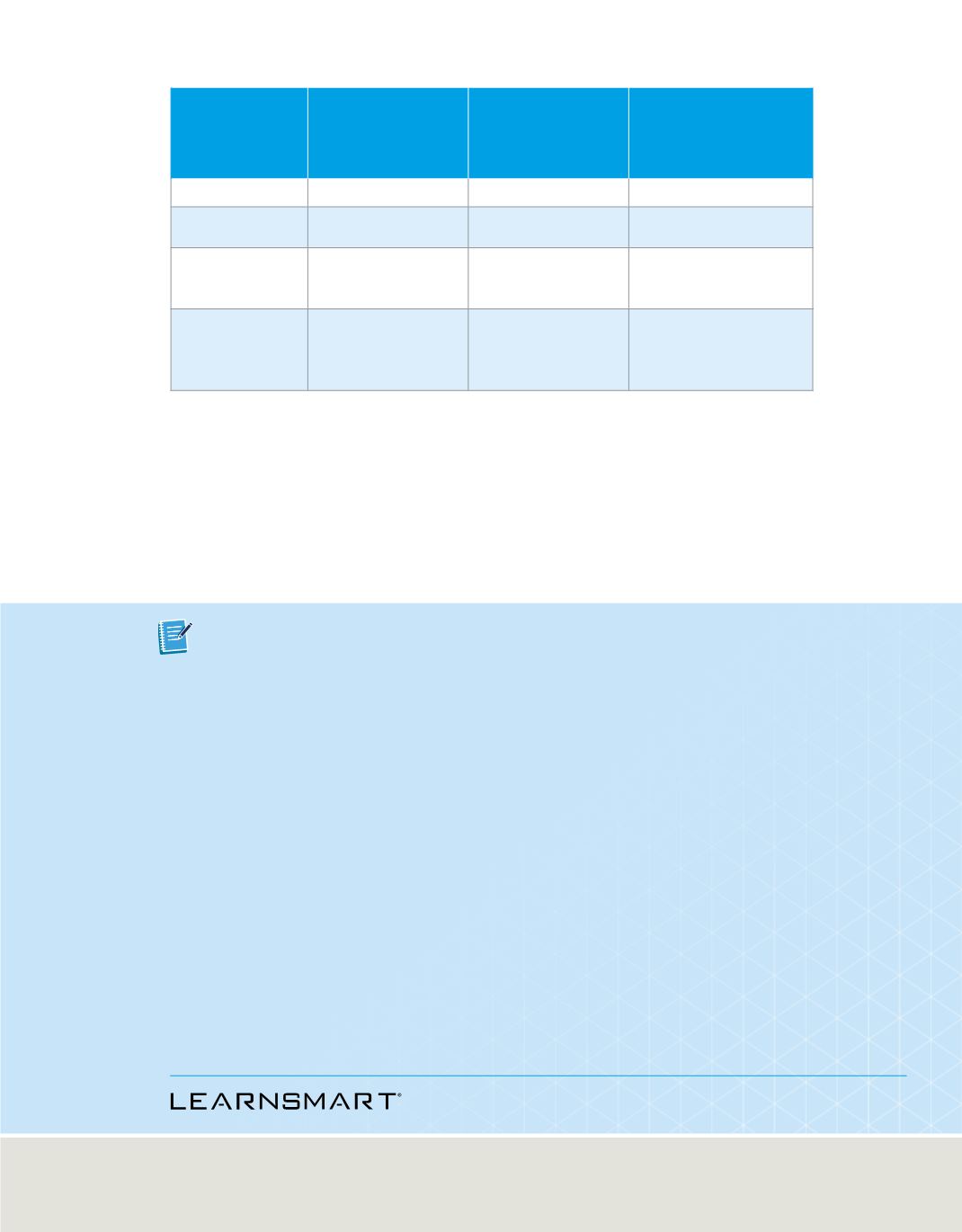
Hydrogen’s first four principal energy levels, sublevels, and related atomic orbitals are
summarized in
Table 2
. Note that the number of orbitals related to each sublevel is
always an odd number, and that the maximum number of orbitals related to each
principal energy level equals
n
2
.
At any given time, the electron in a hydrogen atom can occupy just one orbital. You can
think of the other orbitals as unoccupied spaces—spaces available should the atom’s
energy increase or decrease.
Summary
• Bohr’s atomic model attributes
hydrogen’s emission spectrum
to electrons dropping from
higher-energy to lower-energy
orbits.
• The de Broglie equation relates
a particle’s wavelength to its
mass, its velocity, and Planck’s
constant.
• The quantum mechanical
model assumes that electrons
have wave properties.
• Electrons occupy three-dimensional regions of space
called atomic orbitals.
Check Your Progress
Demonstrate Understanding
13.
Explain
the reason, according to Bohr’s atomic
model, why atomic emission spectra contain only
certain frequencies of light.
14.
Differentiate
between the wavelength of visible light
and the wavelength of a moving soccer ball.
15.
Explain
why the location of an electron in an atom is
uncertain using the Heisenberg uncertainty principle.
How is the location of electrons in atoms defined?
16.
Compare and contrast
Bohr’s model and the
quantum mechanical model of the atom.
17.
Enumerate
the sublevels contained in the hydrogen
atom’s first four energy levels. What orbitals are
related to each s sublevel and each p sublevel?
18.
Calculate
Use the information in
Table 1
to calculate
how many times larger the hydrogen atom’s seventh
Bohr radius is than its first Bohr radius.
Table 2
Hydrogen’s First Four Principal Energy Levels
Principal
Quantum
Number (
n
)
Sublevels (Types
of Orbitals)
Present
Number of
Orbitals Related
to Sublevel
Total Number of
Orbitals Related to
Principal Energy
Level (
n
2
)
1
s
1
1
2
s
p
1
3
4
3
s
p
d
1
3
5
9
4
s
p
d
f
1
3
5
7
16
Go online to follow your personalized learning path to review, practice,
and reinforce your understanding.
Lesson 2 • Quantum Theory and the Atom
125









