
C05_031A
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
→
1.
4.
2.
5.
3.
6.
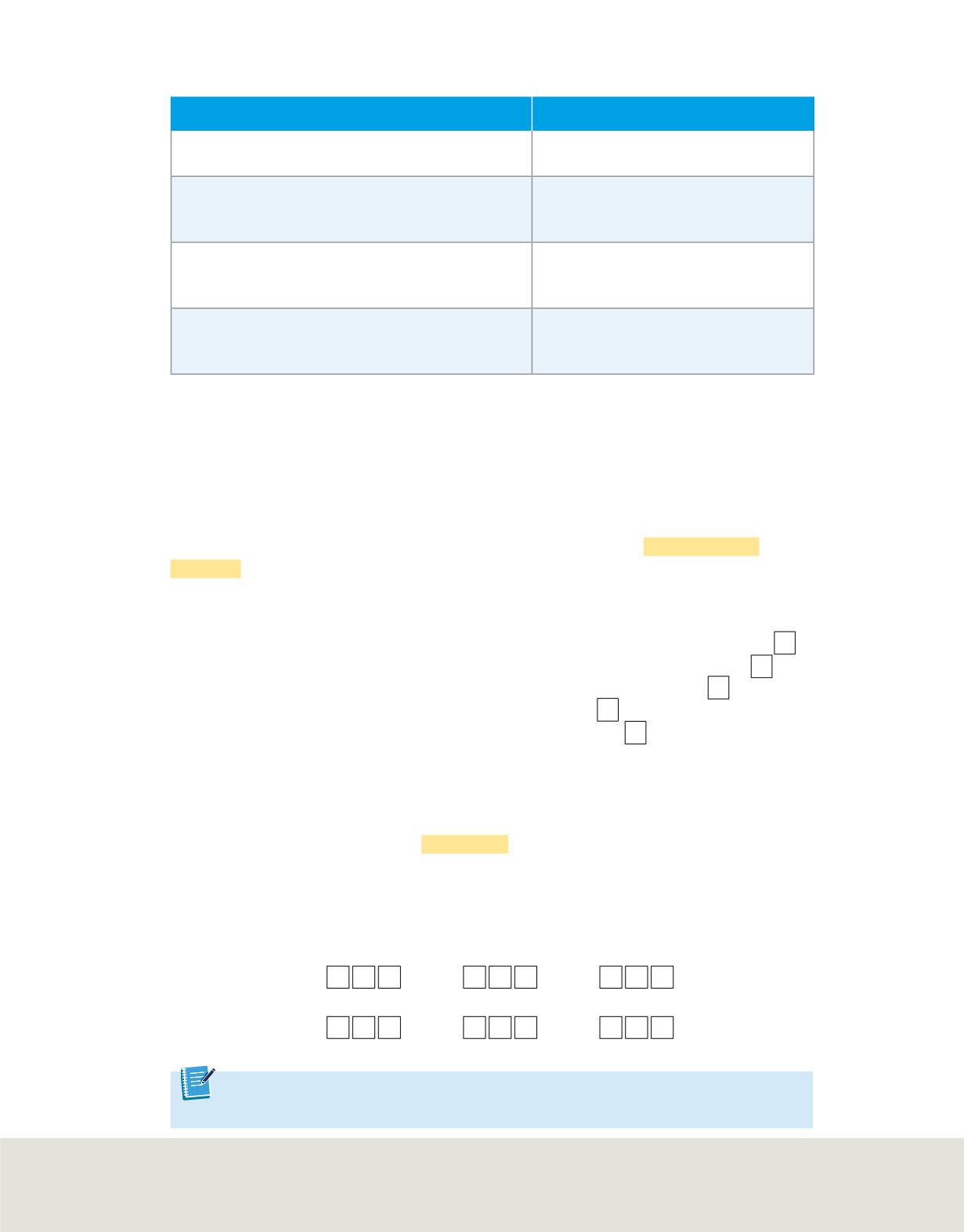
Table 3
summarizes several features of the aufbau diagram. Although the aufbau
principle describes the sequence in which orbitals are filled with electrons, it is
important to know that atoms are not built up electron by electron.
The Pauli exclusion principle
Every electron has an associated spin, similar to the way a top spins on its point. Like a
top, an electron is able to spin in only one of two directions. The
Pauli exclusion
principle,
proposed by Austrian physicist Wolfgang Pauli (1900–1958), states that a
maximum of two electrons can occupy a single atomic orbital, but only if the electrons
have opposite spins.
Electrons in orbitals can be represented by arrows in boxes. An arrow pointing up
↑
represents the electron spinning in one direction, and an arrow pointing down
↓
represents the electron spinning in the opposite direction. An empty box represents
an unoccupied orbital, a box containing a single up arrow
↑
represents an orbital with
one electron, and a box containing both up and down arrows
↑↓
represents a filled
orbital containing a pair of electrons with opposite spins.
Hund’s rule
The fact that negatively charged electrons repel each other affects the distribution of
electrons in equal-energy orbitals.
Hund’s rule
states that single electrons with the same
spin must occupy each equal-energy orbital before additional electrons with opposite
spins can occupy the same orbitals. For example, the boxes below show the sequence
in which six electrons occupy the three 2p orbitals. One electron enters each of the
orbitals before a second electron enters any of the orbitals.
Get It?
State
the three rules that define how electrons are arranged in atoms.
Table 3
Features of the Aufbau Diagram
Feature
Example
All orbitals related to an energy sublevel are of
equal energy.
All three 2p orbitals are of equal
energy.
In a multi-electron atom, the energy sublevels
within a principal energy level have different
energies.
The three 2p orbitals are of higher
energy than the 2s orbital.
In order of increasing energy, the sequence of
energy sublevels within a principal energy level is
s, p, d, and f.
If
n
= 4, then the sequence of energy
sublevels is 4s, 4p, 4d, and 4f.
Orbitals related to energy sublevels within one
principal energy level can overlap orbitals related
to energy sublevels within another principal level.
The orbital related to the atom’s 4s
sublevel has a lower energy than the
five orbitals related to the 3d sublevel.
Lesson 3 • Electron Configuration
127









