
C05_019A
C05_020A
Energy of hydrogen atom
1
2
3
4
5
6
∞
n
As a result of this transition, the atom emits a photon corresponding to the energy
difference between the two levels.
∆
E
=
E
higher-energy orbit
-
E
lower-energy orbit
=
E
photon
=
h
ν
Because only certain atomic energies are possible, only certain frequencies of electromag-
netic radiation can be emitted.
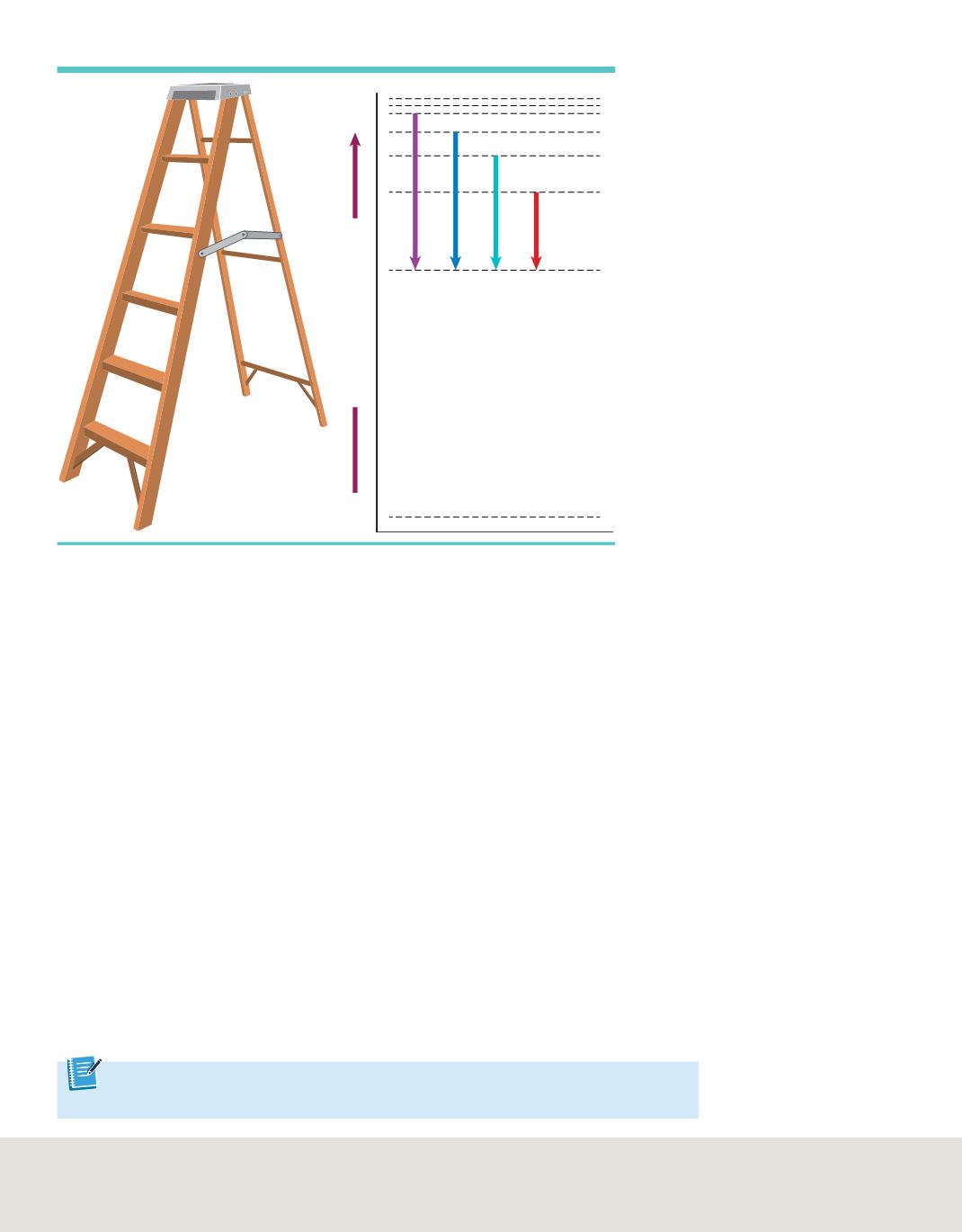
You might compare hydrogen’s atomic energy states to rungs on a ladder, as shown in
Figure 12
. A person can climb up or down the ladder only from rung to rung. Similarly,
the hydrogen atom’s electron can move only from one allowable orbit to another, and
therefore, can emit or absorb only certain amounts of energy, corresponding to the energy
difference between the two orbits. Unlike rungs on a ladder, however, the hydrogen
atom’s energy levels are not evenly spaced.
Figure 12
also illustrates the four electron transitions that account for visible lines in
hydrogen’s atomic emission spectrum, shown in
Figure 8
. Electron transitions from
higher-energy orbits to the second orbit account for all of hydrogen’s visible lines, which
form the Balmer series. Other electron transitions have been measured that are not
visible, such as the Lyman series (ultraviolet), in which electrons drop into the
n
=
1 orbit,
and the Paschen series (infrared), in which electrons drop into the
n
=
3 orbit.
Figure 12
Only certain energy levels are allowed. The energy levels are similar to the rungs of
a ladder. The four visible lines correspond to electrons dropping from a higher
n
to the orbit
n
=
2. As
n
increases, the hydrogen atom’s energy levels are closer to each other.
Get It?
Explain
why different colors of light result from electron behavior in the atom.
118
Module 4 • Electrons in Atoms









