
C05_028A
z
x
x
y
y
1s orbital
z
2s orbital
z
x
x
y
y
z
p
x
y
p
y
z
x
p
z
C05_029A
y
y
y
y
x
z
y
z
x
x
x
x
z
d
xz
d
xy
z
d
yz
d
x
2
y
2
d
z
2
z
c
b
a
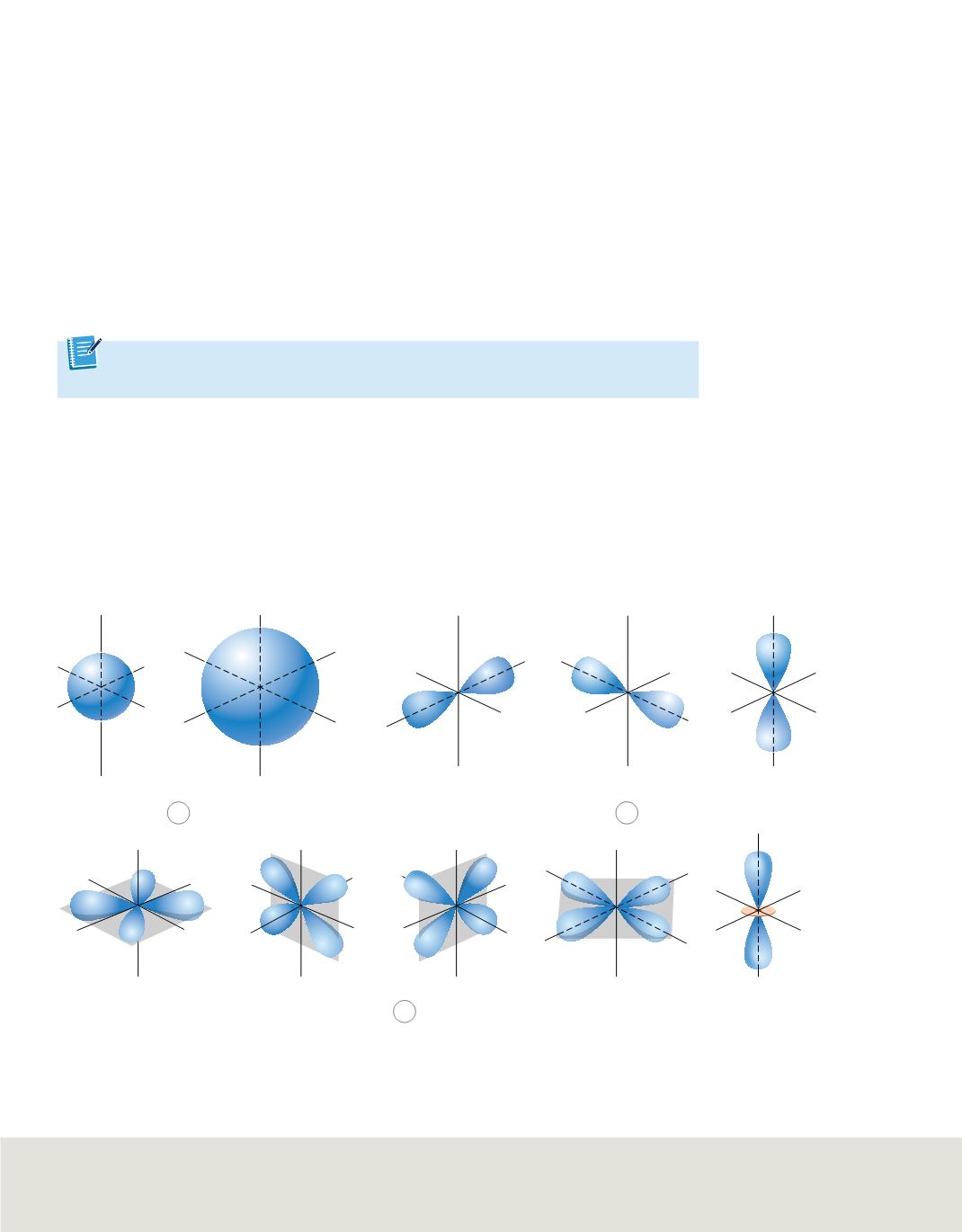
Shapes of orbitals
Sublevels are labeled
s, p, d,
or f
according to the shapes of the atom’s orbitals. All s
orbitals are spherical, and all p orbitals are dumbbell-shaped; however, not all d or f
orbitals have the same shape. Each orbital can contain, at most, two electrons. The
single sublevel in principal energy level 1 corresponds to a spherical orbital called the
1s orbital. The two sublevels in principal energy level 2 are designated 2s and 2p. The
2s sublevel corresponds to the 2s orbital, which is spherical like the 1s orbital but larger
in size, as shown in
Figure 17a
. The 2p sublevel corresponds to three dumbbell-shaped
p orbitals designated 2p
x
, 2p
y
, and 2p
z
. The subscripts
x
,
y
, and
z
merely designate the
orientations of p orbitals along the
x
,
y
, and
z
coordinate axes, as shown in
Figure 17b
.
Each of the p orbitals related to an energy sublevel has the same energy.
Get It?
Describe
the shapes of
s
and
p
orbitals.
Principal energy level 3 consists of three sublevels designated 3s, 3p, and 3d. Each d
sublevel relates to five orbitals of equal energy. Four of the d orbitals have identical
shapes but different orientations along the
x
,
y
, and
z
coordinate axes. However, the
fifth orbital, d
z
, is shaped and oriented differently than the other four. The shapes and
orientations of the five d orbitals are illustrated in
Figure 17c
. The fourth principal
energy level (
n
=
4) contains a fourth sublevel, called the 4f sublevel, which relates to
seven f orbitals of equal energy. The f orbitals have complex, multilobed shapes.
Figure 17
The shapes of atomic orbitals describe the probable distribution of electrons in energy sublevels.
a.
All s orbitals are spherical, and their size increases with increasing principal quantum number.
b.
The three p orbitals are dumbbell-shaped and are oriented along the three perpendicular
x
,
y
, and
z
axes.
c.
Four of the five d orbitals have the same shape but lie in different planes. The
d
z
2
orbital has its own
unique shape.
124
Module 4 • Electrons in Atoms









