
C05_025A
Nucleus
Boundary
Density Maps
a
b
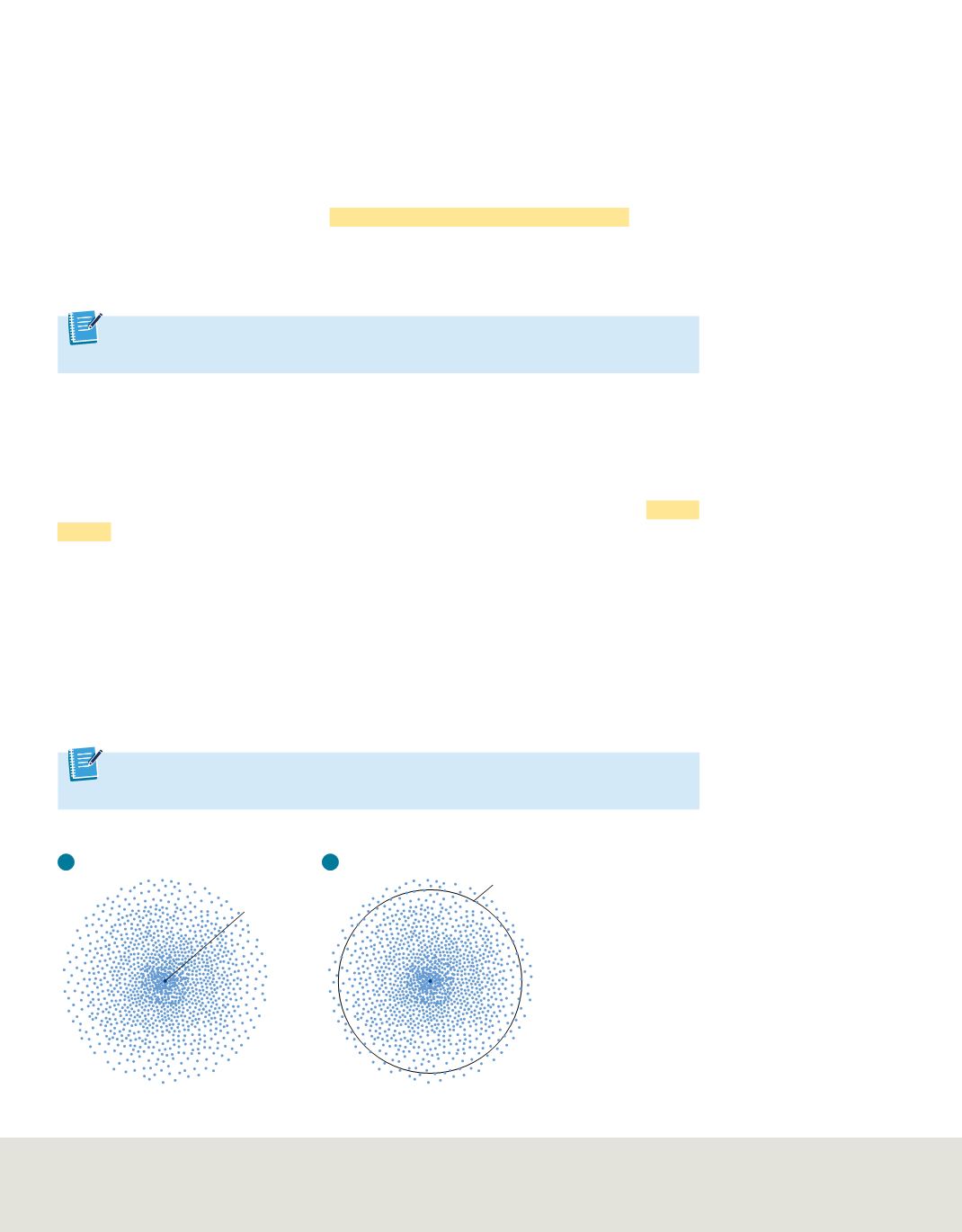
Figure 15
The density map
represents the probability of finding
an electron at a given position
around the nucleus.
a.
The higher
density of points near the nucleus
shows that the electron is more
likely to be found close to the
nucleus.
b.
At any given time, there
is a 90% probability of finding the
electron within the circular region
shown. This surface is sometimes
chosen to represent the boundary
of the atom. In this illustration, the
circle corresponds to a projection of
the 3-dimensional sphere that
contains the electrons.
The Schrödinger wave equation
In 1926, Austrian physicist Erwin Schrödinger (1887–1961) furthered the wave-particle
theory proposed by de Broglie. Schrödinger derived an equation that treated the
hydrogen atom’s electron as a wave. Schrödinger’s new model for the hydrogen atom
seemed to apply equally well to atoms of other elements—an area in which Bohr’s model
failed. The atomic model in which electrons are treated as waves is called the wave
mechanical model of the atom or the
quantum mechanical model of the atom.
Like
Bohr’s model, the quantum mechanical model limits an electron’s energy to certain
values. However, unlike Bohr’s model, the quantum mechanical model makes no
attempt to describe the electron’s path around the nucleus.
Electron’s probable location
The Schrödinger wave equation is too complex to be considered here. However, each
solution to the equation is known as a wave function, which is related to the probability
of finding the electron within a particular volume of space around the nucleus. The
wave function predicts a three-dimensional region around the nucleus, called an
atomic
orbital,
which describes the electron’s probable location. An atomic orbital is like a
fuzzy cloud in which the density at a given point is proportional to the probability of
finding the electron at that point.
Figure 15a
illustrates the probability map that describes the electron in the atom’s
lowest energy state. The probability map can be thought of as a time-exposure
photograph of the electron moving around the nucleus, in which each dot represents
the electron’s location at an instant in time. The high density of dots near the nucleus
indicates the electron’s most probable location. However, it is also possible that the
electron might be found at a considerable distance from the nucleus.
Get It?
Compare and contrast
Bohr’s model and the quantum mechanical model.
Get It?
Describe
where electrons are located in an atom.
122
Module 4 • Electrons in Atoms









