
C05_018A
Ultraviolet
series
(Lyman)
Visible series
(Balmer)
n
=
7
n
=
6
Infrared
series
(Paschen)
n
=
5
n
=
4
n
=
3
n
=
1
+
n
=
2
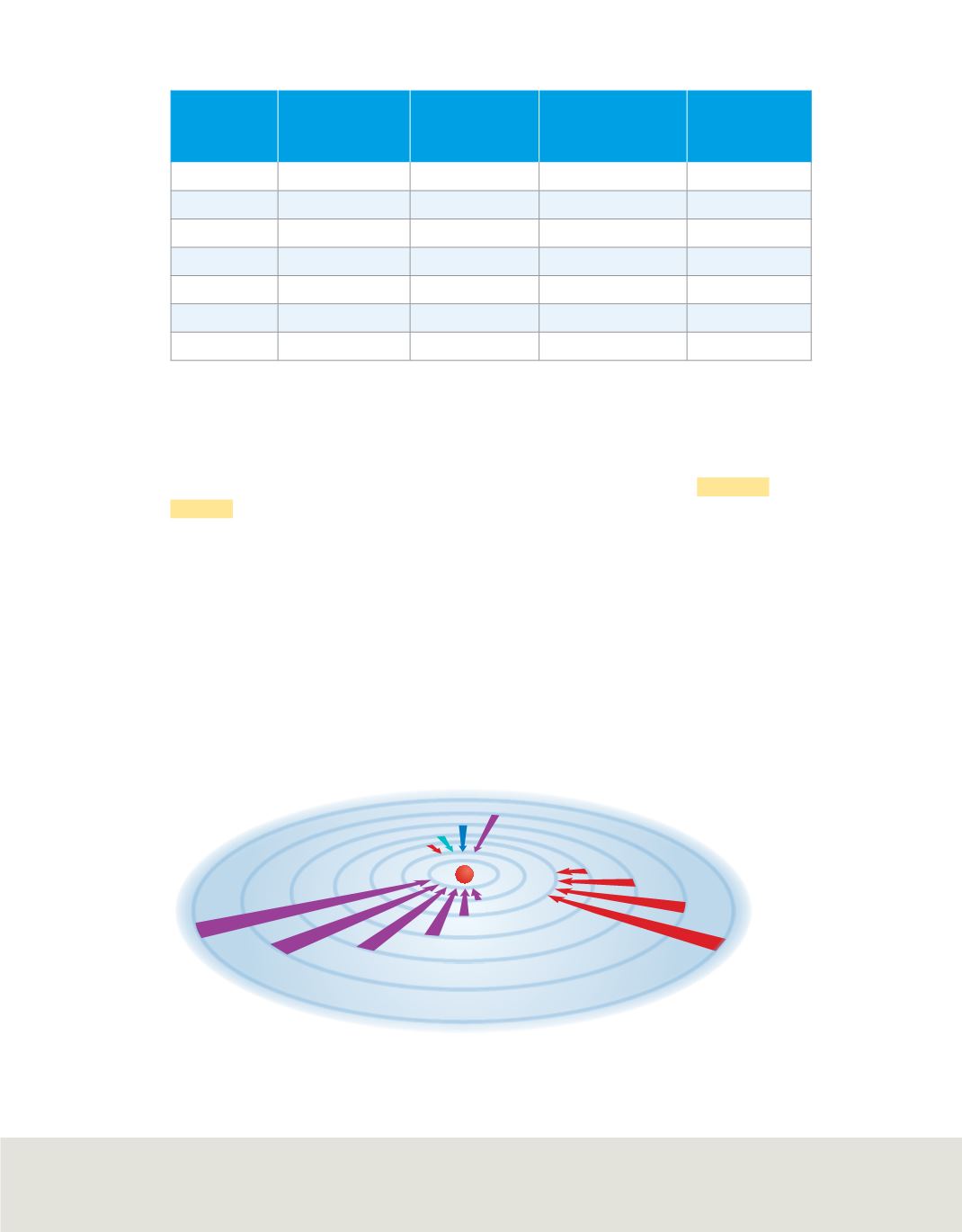
Bohr suggested that the electron in a hydrogen atom moves around the nucleus in only
certain allowed circular orbits. The smaller the electron’s orbit, the lower the atom’s
energy state, or energy level. Conversely, the larger the electron’s orbit, the higher the
atom’s energy state, or energy level. Bohr assigned a number,
n
, called a
quantum
number,
to each orbit. He also calculated the radius of each orbit.
Table 1
shows data
for the first seven energy levels of a hydrogen atom according to Bohr’s model.
The hydrogen line spectrum
Bohr suggested that a hydrogen atom is in the ground state when its single electron is in
the
n
=
1 orbit, also called the first energy level. In the ground state, the atom does not
radiate energy. When energy is added from an outside source, the electron moves to a
higher-energy orbit, putting the atom in an excited state. When the atom is in an excited
state, the electron can drop from the higher-energy orbit to a lower-energy orbit, as
shown in
Figure 11.
Table 1
Bohr’s Description of the Hydrogen Atom
Bohr’s
Atomic
Orbit
Quantum
Number
Orbit Radius
(nm)
Corresponding
Atomic Energy
Level
Relative
Energy
First
n
=
1
0.0529
1
E
1
Second
n
=
2
0.212
2
E
2
=
4
E
1
Third
n
=
3
0.476
3
E
3
=
9
E
1
Fourth
n
=
4
0.846
4
E
4
=
16
E
1
Fifth
n
=
5
1.32
5
E
5
=
25
E
1
Sixth
n
=
6
1.90
6
E
6
=
36
E
1
Seventh
n
=
7
2.59
7
E
7
=
49
E
1
Figure 11
When an electron drops from a higher-energy orbit to a lower-energy orbit, a photon is
emitted. The ultraviolet (Lyman), visible (Balmer), and infrared (Paschen) series correspond to
electrons dropping to
n
=
1,
n
=
2, and
n
=
3, respectively.
Lesson 2 • Quantum Theory and the Atom
117









