
Brian Kanof/McGraw-Hill Education
1 half–wavelength
n
=
3 wavelengths
n
≠
whole number (not allowed)
n
=
5 wavelengths
2 half–wavelengths
3 half–wavelengths
Vibrating harp string
Orbiting electron
c
b
n
=
1
n
=
2
n
=
3
B
A
B
A
B
A
1 half–wavelength
n
=
3 wavelengths
n
≠
whol number (not allowed)
n
=
5 wavelengths
2 half–wavelengths
3 half–wavelengths
Vibrating harp string
Orbiting electron
c
b
n
=
1
n
=
2
n
=
3
B
A
B
A
B
A
a
The limits of Bohr’s model
Bohr’s model explained hydrogen’s observed spectral lines. However, the model failed
to explain the spectrum of any other element. Moreover, Bohr’s model did not fully
account for the chemical behavior of atoms. In fact, although Bohr’s idea of quantized
energy levels laid the groundwork for atomic models to come, later experiments
demonstrated that the Bohr model was fundamentally incorrect. The movements of
electrons in atoms are not completely understood even now; however, substantial
evidence indicates that electrons do not move around the nucleus in circular orbits.
The Quantum Mechanical Model of the Atom
Scientists in the mid-1920s, convinced that the Bohr atomic model was incorrect,
formulated new and innovative explanations of how electrons are arranged in atoms.
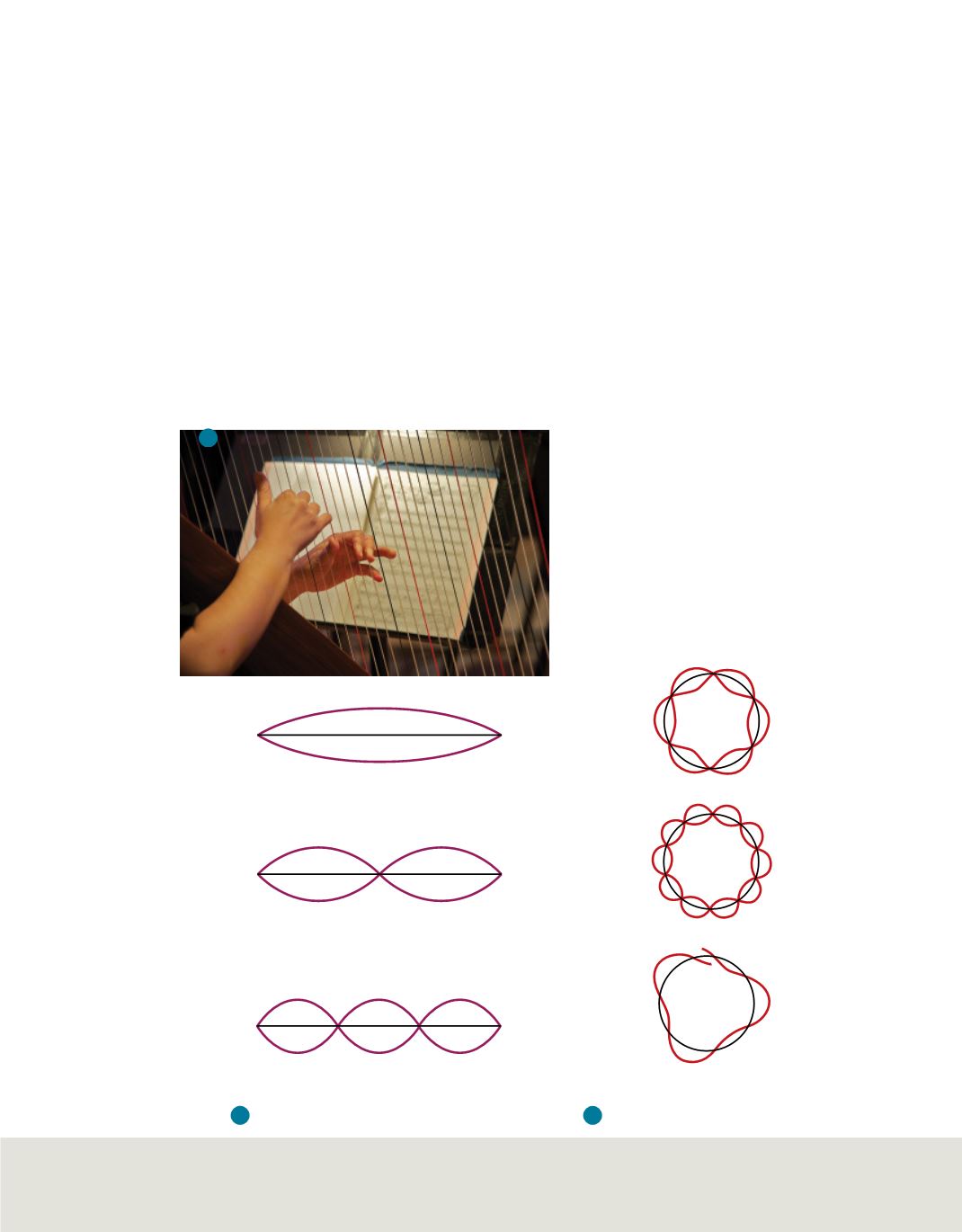
In 1924, a French graduate student in physics named Louis de Broglie (1892–1987)
proposed a new idea, shown in
Figure 13
and discussed on the following page
.
Figure 13 a.
The string on the
harp vibrates between two fixed
endpoints.
b.
The vibrations of a
string between the two fixed
endpoints labeled A and B are
limited to multiples of half-
wavelengths.
c.
Electrons on
circular orbits can only have
whole numbers of wavelengths.
Lesson 2 • Quantum Theory and the Atom
119









