
STEM CAREER Connection
Astrochemist
Do you like chemistry, planetary science, chemical biology, physics, astronomy,
and computational science? A career in astrochemisty may be the career for you.
Astrochemists use telescopes, satellites, and space vehicles to collect spectro-
scopic data and analyze it. In this career, knowledge from several scientific
disciplines is used to analyze and model the data collected.
ACADEMIC VOCABULARY
phenomenon
an observable fact or event
During
rainstorms, electric currents often pass
from the sky to Earth—a phenomenon
called lightning.
114
Module 4 • Electrons in Atoms
C05_015A
400
λ
(nm)
434
nm
486
nm
656
nm
Prism separates light
into its components.
Hydrogen gas
discharge tube
emits light.
410
nm
450
Hydrogen’s Atomic Emission Spectrum
750
500 550 600 650 700
Slit
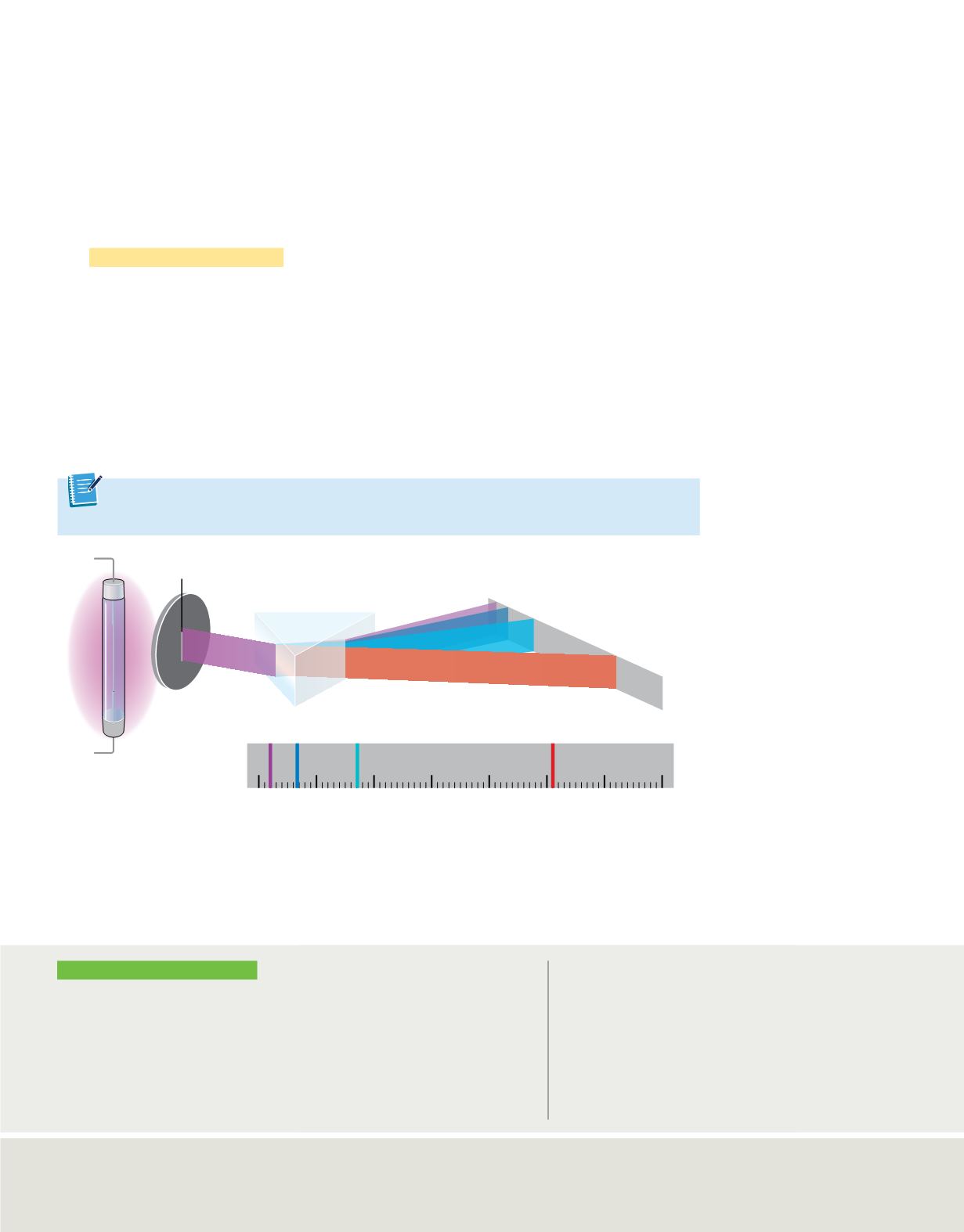
Atomic Emission Spectra
Have you ever wondered how light is produced in the glowing tubes of neon signs? This
is another phenomenon that cannot be explained by the wave model of light. The light
of a neon sign is produced by passing electricity through a tube filled with neon gas.
Neon atoms in the tube absorb energy and become excited. These excited atoms return
to their stable state by emitting light to release that energy. If the light emitted by the
neon is passed through a glass prism, neon’s atomic emission spectrum is produced.
The
atomic emission spectrum
of an element is the set of frequencies of the
electromagnetic waves emitted by atoms of the element.
Figure 8
shows the purple-pink
glow produced by excited hydrogen atoms and the visible portion of hydrogen’s
emission spectrum responsible for producing the glow. Note that an atomic emission
spectrum is not a continuous spectrum. Rather, it consists of several individual lines of
color corresponding to the frequencies of radiation emitted by the atoms.
Each element’s atomic emission spectrum is unique and can be used to identify an
element. For example, when a platinum wire is dipped into a strontium nitrate solution
and then held in a burner flame, the strontium atoms emit a characteristic red color.
Get It?
Explain
how an emission spectrum is produced.
Figure 8
The purple light emitted by hydrogen can be separated into its different components using a
prism. Hydrogen has an atomic emission spectrum that comprises four lines of different wavelengths.
Determine
Which line has the highest energy?









