
C05_016A
400 nm
400 nm
700 nm
700 nm
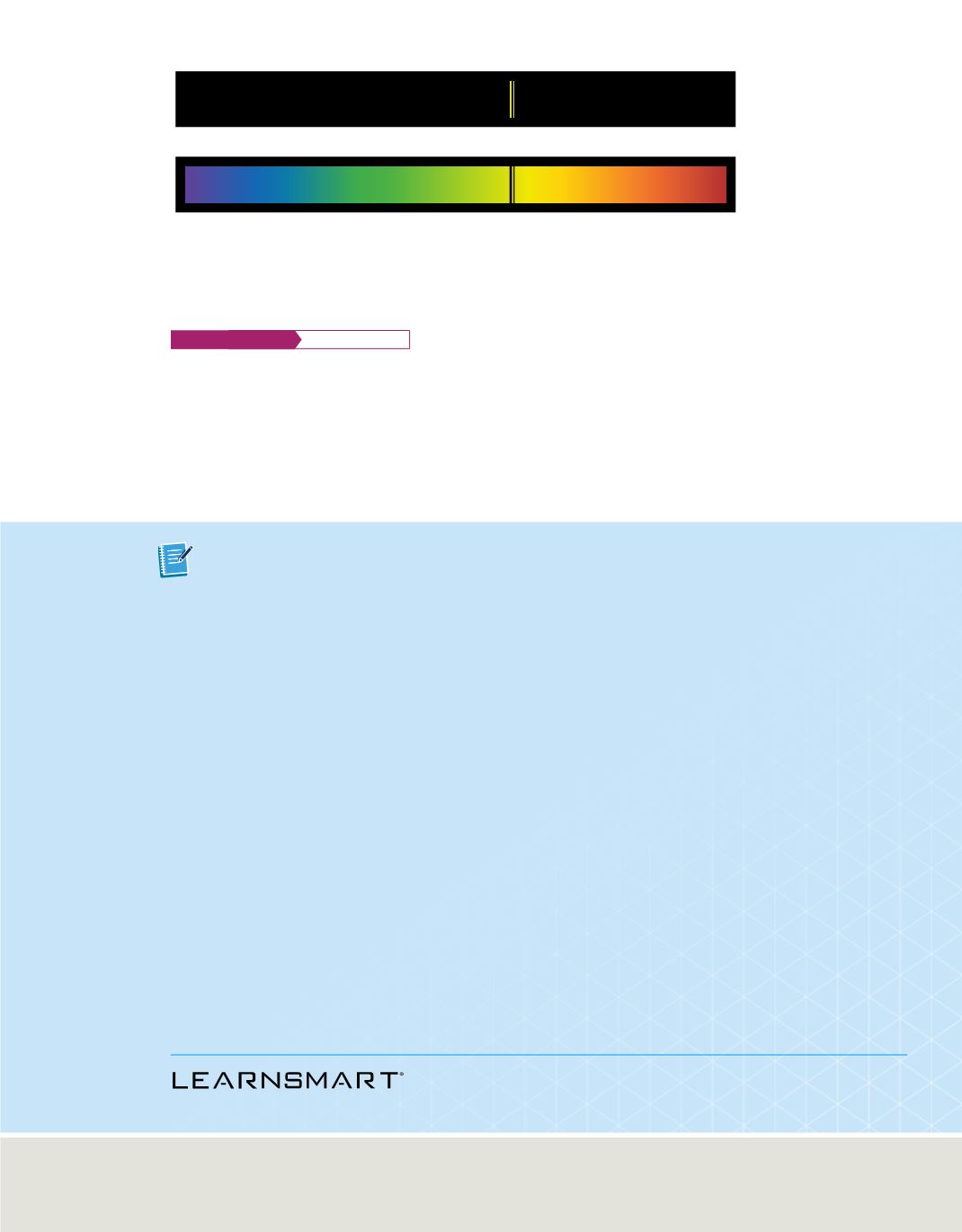
Sodium absorption spectrum
Sodium emission spectrum
Figure 9
When excited sodium atoms return to a less excited state, they emit light at certain frequencies,
producing an emission spectrum. When a continuous spectrum of light passes through sodium gas, atoms in
the gas absorb light at those same frequencies, producing an absorption spectrum with dark spectral lines.
Go online to follow your personalized learning path to review, practice,
and reinforce your understanding.
Summary
• All waves are defined by their
wavelengths, frequencies,
amplitudes, and speeds.
• In a vacuum, all electromag-
netic waves travel at the speed
of light.
• All electromagnetic waves
have both wave and particle
properties.
• Matter emits and absorbs
energy in quanta.
• White light produces a continu-
ous spectrum. An element’s
emission spectrum consists of
a series of lines of individual
colors.
Check Your Progress
ASTRONOMY
Connection
Astronomers use atomic spectra to determine the
composition of the outer layers of stars. When a continuous spectrum of light from
within a star passes through the outer layers of the star, atoms in the outer layers absorb
light at certain frequencies, producing an absorption spectrum. The lines in the absorp-
tion spectrum reveal what elements are in the outer layers of the star because the
frequencies absorbed in an element’s absorption spectrum are the same as those
emitted in the element’s emission spectrum, as shown for sodium in
Figure 9.
Demonstrate Understanding
8.
Describe
the relationship between changing electric
and magnetic fields and particles.
9.
Compare and contrast
continuous spectrum and
emission spectrum.
10.
Discuss
the way in which Einstein utilized Planck’s
quantum concept to explain the photoelectric effect.
11.
Calculate
Heating 235 g of water from 22.6°C to 94.4°C
in a microwave oven requires 7.06
×
10
4
J of energy. If
the microwave frequency is 2.88
×
10
10
s
-
1
, how many
quanta are required to supply the 7.06
×
10
4
J?
12.
Interpret Scientific Illustrations
Use
Figure 5
and
your knowledge of electromagnetic radiation to match
the numbered items with the lettered items. The
numbered items may be used more than once or
not at all.
a. longest wavelength 1. gamma rays
b. highest frequency
2. ultraviolet light
c. greatest energy
3. radio waves
Lesson 1 • Light and Quantized Energy
115









