
C05_014A
Electrons
Electron ejected
from surface
Metal surface
Incident beam of light
A useful analogy is that of a child building a wall with wooden blocks. The child can
add to or take away from the wall only in increments of whole numbers of blocks.
Similarly, matter can have only certain amounts of energy—quantities of energy between
these values do not exist.
Planck and other physicists of the time thought the concept of quantized energy was
revolutionary, and some found it disturbing. Prior experience had led scientists to think
that energy could be absorbed and emitted in continually varying quantities, with no
minimum limit to the amount. For example, think about heating a cup of water in a
microwave oven. It seems that you can add any amount of thermal energy to the water by
regulating the power and duration of the microwaves. Instead, the
water’s temperature increases in infinitesimal steps as its molecules
absorb quanta of energy. Because these steps are so small, the
temperature seems to rise in a continuous, rather than a
stepwise, manner.
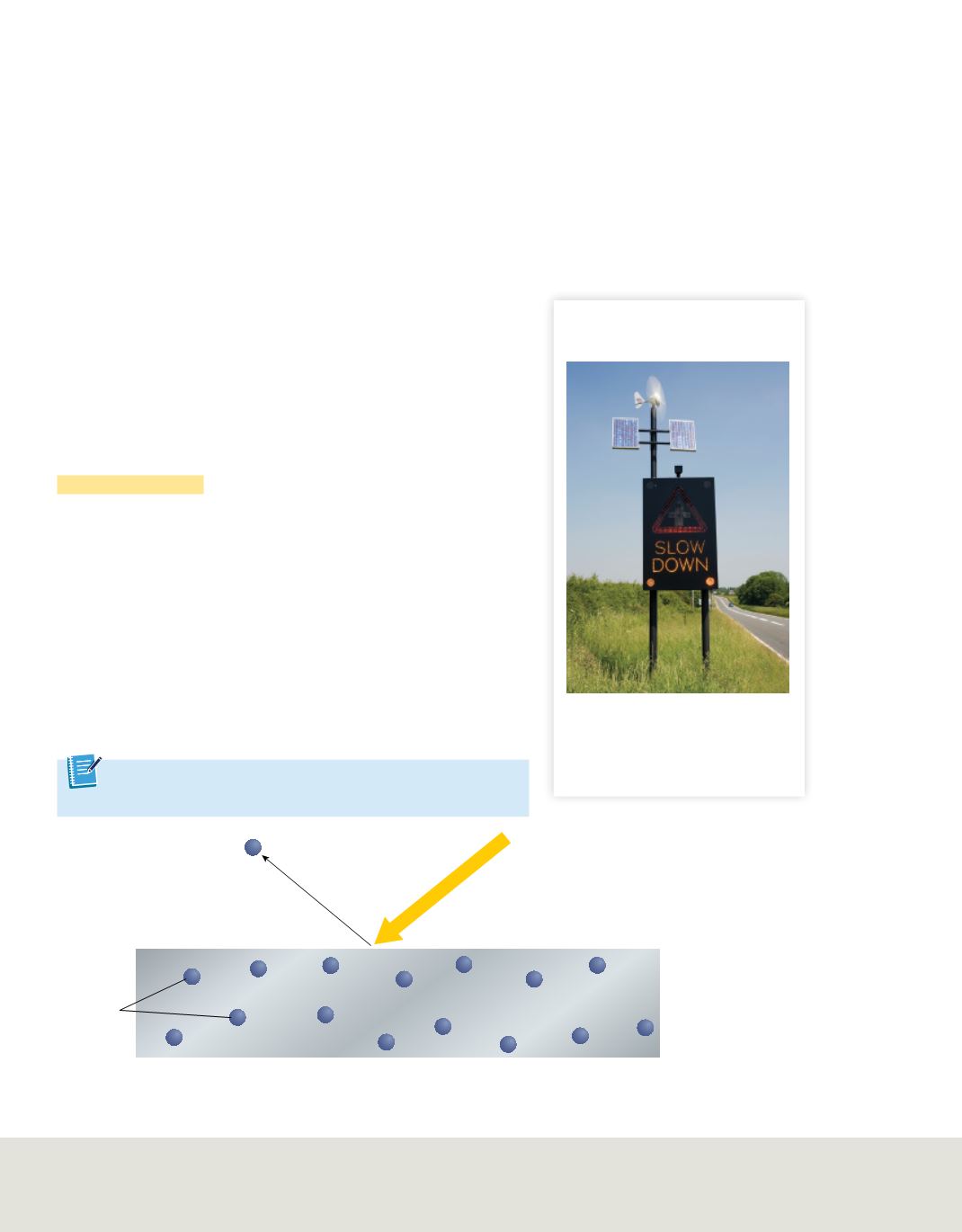
The photoelectric effect
Scientists also knew that the wave model of light could not
explain a phenomenon called the photoelectric effect. In the
photoelectric effect,
electrons, called photoelectrons, are emitted
from a metal’s surface when light at or above a certain frequency
shines on the surface, as shown in
Figure 7
.
The wave model predicts that given enough time, even
low-energy, low-frequency light would accumulate and supply
enough energy to eject photoelectrons from a metal. In reality, a
metal will not eject photoelectrons below a specific frequency of
incident light. For example, no matter how intensely or how long
it shines, light with a frequency less than 1.14
×
10
15
Hz does not
eject photoelectrons from silver. But even dim light with a
frequency equal to or greater than 1.14
×
10
15
Hz ejects
photoelectrons from silver.
Get It?
Describe
the photoelectric effect.
Real-World Chemistry
The Photoelectric Effect
SOLAR ENERGY is sometimes used to
power road signs. Photovoltaic cells
use the photoelectric effect to convert
the energy of light into electric energy.
Figure 7
The photoelectric effect occurs when light of a certain frequency strikes a metal surface and
ejects electrons. When the intensity of the light increases, the number of electrons ejected increases.
When the frequency (energy) of the light increases, the energy of the ejected electrons increases.
112
Module 4 • Electrons in Atoms
Andrew Fox/Corbis/Getty Images









