
C05_008A
Shorter
wavelength
Lower frequency
Higher frequency
Longer
wavelength
All electromagnetic waves, including visible light, travel at a speed of 3.00
×
10
8
m/s in a
vacuum. Because the speed of light is such an important and universal value, it is given its
own symbol, c. The speed of light is the product of its wavelength (
λ
) and its frequency (
ν
).
Electromagnetic Wave Relationship
c
= λν
c
is the speed of light in a vacuum.
λ
is the wavelength.
ν
is the frequency.
The speed of light in a vacuum is equal to the product of the wavelength and the frequency.
Although the speed of all electromagnetic waves in a vacuum is the same, waves can have
different wavelengths and frequencies. As you can see from the equation above, wavelength
and frequency are inversely related; in other words, as one quantity increases, the other
decreases. To better understand this
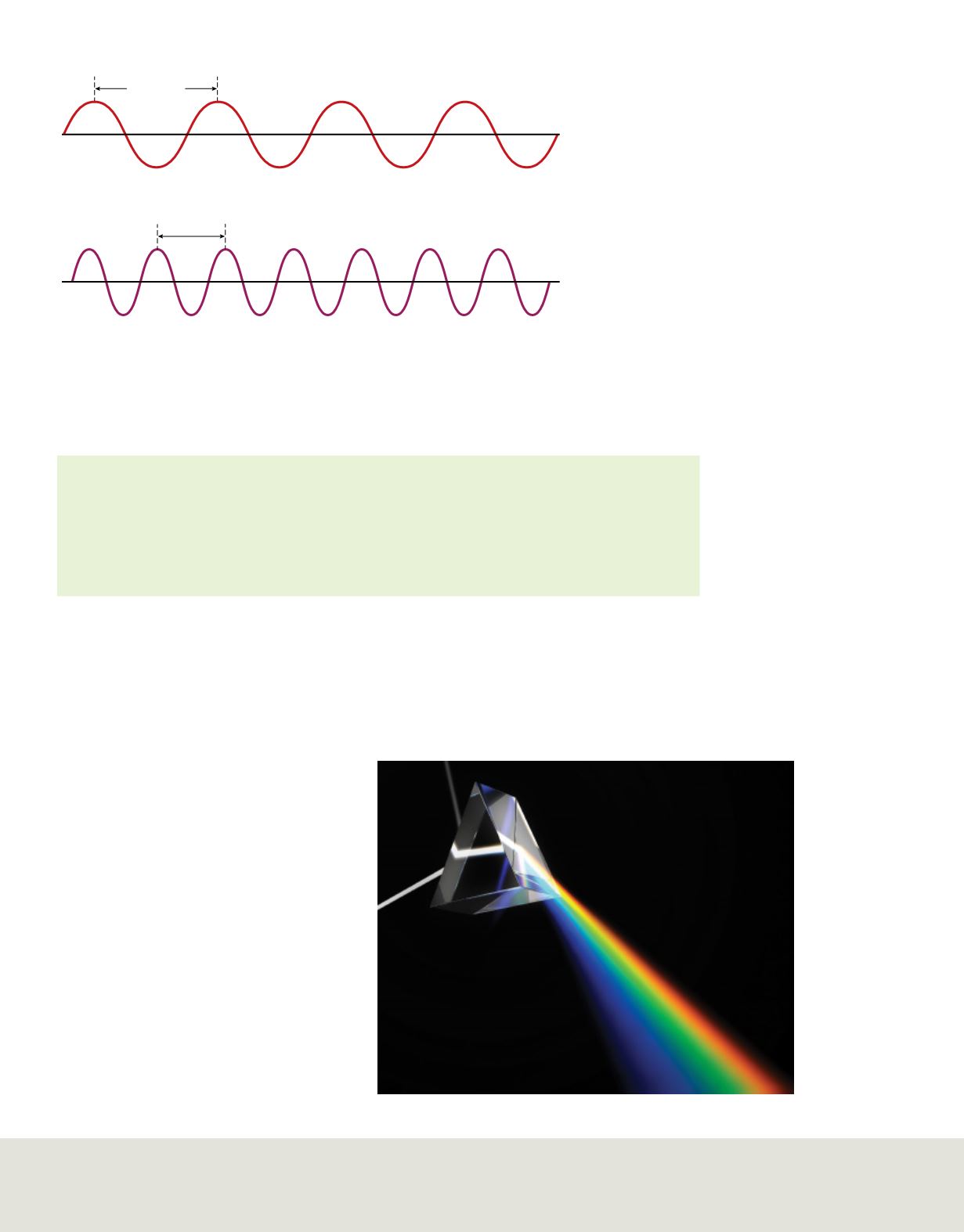
relationship, examine the two waves
illustrated in
Figure 3
. Although both waves
travel at the speed of light, you can see that
the red wave has a longer wavelength and
lower frequency than the violet wave.
Electromagnetic spectrum
Sunlight, which is one example of white
light, contains a nearly continuous range
of wavelengths and frequencies. White
light passing through a prism separates
into a continuous spectrum of colors
similar to the spectrum in
Figure 4
.
These are the colors of the visible
spectrum. The spectrum is called
continuous because each point of it
corresponds to a unique wavelength
and frequency.
Figure 3
These waves illustrate the
relationship between wavelength
and frequency. As frequency
increases, wavelength decreases.
Infer
Does frequency or wavelength
affect amplitude?
Figure 4
When white light passes through a prism, it is separated
into a continuous spectrum of its different components—red, orange,
yellow, green, blue, indigo, and violet light.
108
Module 4 • Electrons in Atoms
Andrzej Wojcicki/Science Photo Library/Alamy









