
C05_007A
Wavelength (
λ
)
Wavelength (
λ
)
Crest
Amplitude
Trough
Origin
b
a
In the early 1900s, scientists began to unravel the puzzle of chemical behavior. They
observed that certain elements emitted visible light when heated in a flame. Analysis of
the emitted light revealed that an element’s chemical behavior is related to the
arrangement of the electrons in its atoms. To understand this relationship and the
nature of atomic structure, it will be helpful to first understand the nature of light.
The Wave Nature of Light
Visible light is a type of
electromagnetic radiation
—a form of energy that exhibits
wavelike behavior as it travels through space. It can be modeled as a wave of changing
electric and magnetic fields. Other examples of electromagnetic radiation include
microwaves, X rays, and television and radio waves.
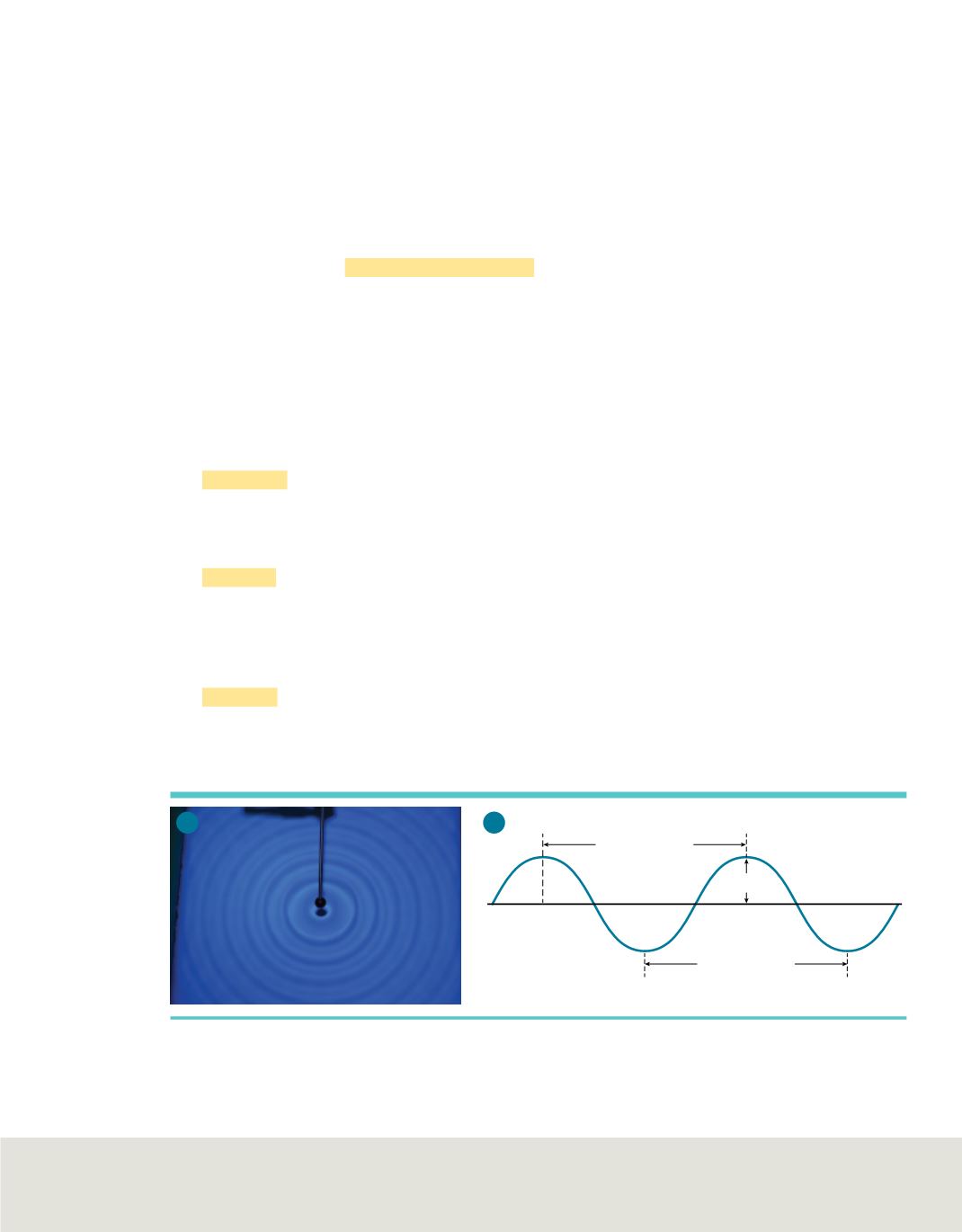
Characteristics of waves
All waves can be described by several characteristics, a few of which might be familiar
to you from everyday experience. You might have seen concentric waves when
dropping an object into water, as shown in
Figure 2a
.
The
wavelength
(represented by
λ
, the Greek letter lambda) is the shortest distance
between equivalent points on a continuous wave. For example, in
Figure 2b,
the
wavelength is measured from crest to crest or from trough to trough. Wavelength is
usually expressed in meters, centimeters, or nanometers (1 nm
=
1
×
10
-
9
m).
The
frequency
(represented by
ν
, the Greek letter nu) is the number of waves that pass
a given point per second. One hertz (Hz), the SI unit of frequency, equals one wave per
second. In calculations, frequency is expressed with units of waves per second, (1/s) or
(s
-
1
); the term waves is understood. A particular frequency can be expressed in the
following ways: 652 Hz
=
652 waves/second
=
652/s
=
652 s
-
1
.
The
amplitude
of a wave is the wave’s height from the origin to a crest, or from the
origin to a trough, as illustrated in
Figure 2b.
Wavelength and frequency do not affect
the amplitude of a wave.
Figure 2 a.
The concentric waves in the water show the characteristic properties of all waves.
b.
Amplitude,
wavelength, and frequency are the main characteristics of waves.
Identify
a crest, a trough, and one wavelength in the photo.
Lesson 1 • Light and Quantized Energy
107
Matt Meadows/McGraw-Hill Education









