
The Particle Nature of Light
While considering light as a wave explains much of its everyday behavior, it fails to
adequately describe important aspects of light’s interactions with matter. The wave
model of light cannot explain why heated objects emit only certain frequencies of light
at a given temperature, or why some metals emit electrons when light of a specific
frequency shines on them. Scientists realized that a new model or a revision of the wave
model of light was needed to address these phenomena.
The quantum concept
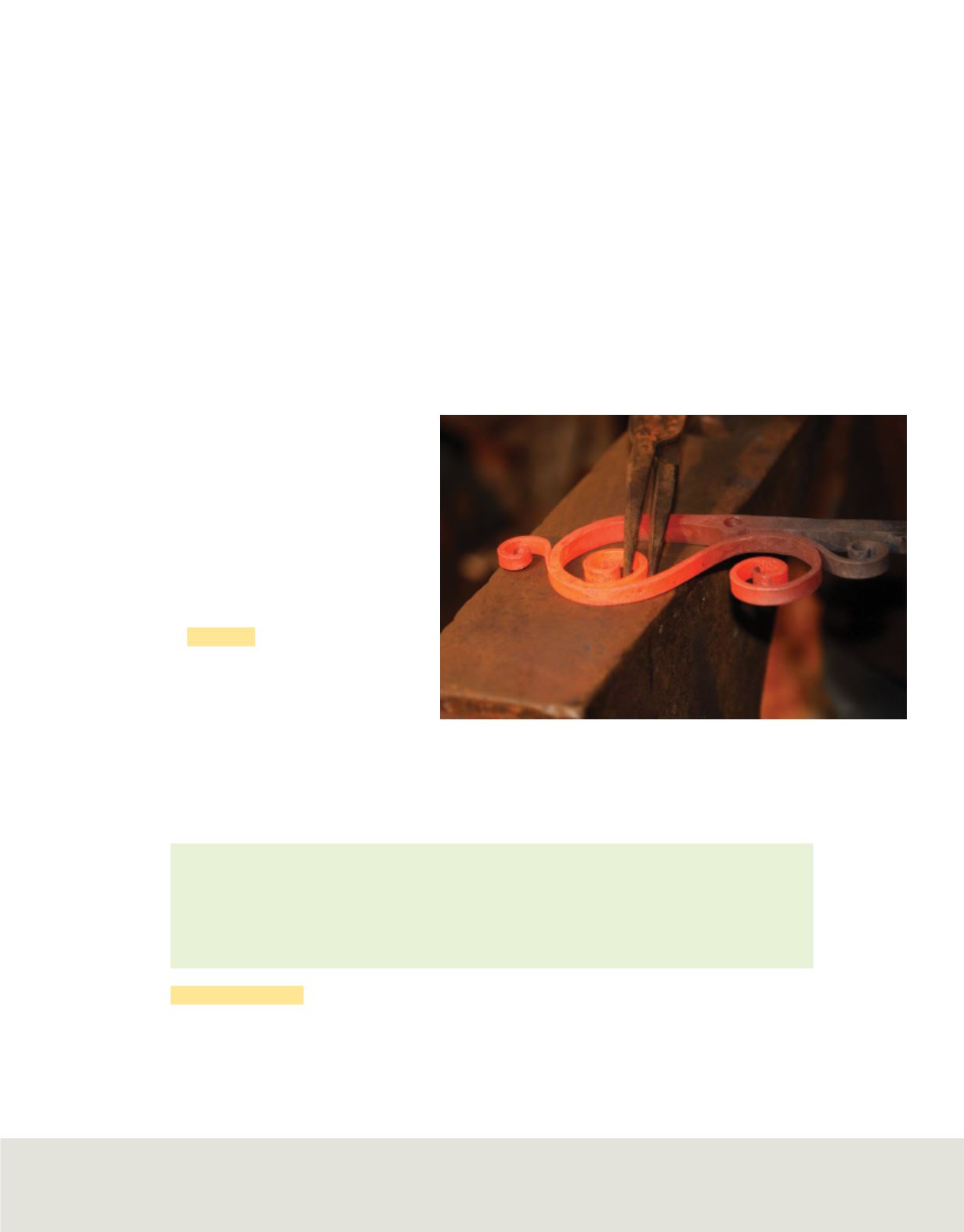
When objects are heated, they emit glowing light.
Figure 6
illustrates this phenomenon
with iron. A piece of iron appears dark gray at room temperature, glows red when
heated sufficiently, and turns orange, then bluish in color at even higher temperatures.
As the iron gets hotter, it has more energy and emits different colors of light. These
different colors correspond to different frequencies and wavelengths.
The wave model could not explain
the emission of these different
wavelengths. In 1900, German
physicist Max Planck (1858–1947)
began searching for an explana-
tion of this phenomenon. His
study led him to a startling
conclusion: matter can gain
or lose energy only in small,
specific amounts called quanta.
A quantum
is the minimum
amount of energy that can be
gained or lost by an atom.
Planck proposed that the energy
emitted by hot objects was quan-
tized. He also showed that there
is a direct relationship between
the energy of a quantum and the
frequency of emitted radiation.
Energy of a Quantum
E
quantum
=
h
ν
E
quantum
represents energy.
h
is Planck’s constant.
ν
represents frequency.
The energy of a quantum is given by the product of Planck’s constant and the frequency.
Planck’s constant,
h
, has a value of 6.626
×
10
-
34
J⋅s, where J is the symbol for joule, the
SI unit of energy. The equation shows that the energy of radiation increases as the
radiation’s frequency,
ν
, increases. According to Planck’s theory, for a given frequency,
ν
, matter can emit or absorb energy only in whole-number multiples of
h
ν
; that is, 1
h
ν
,
2
h
ν
, 3
h
ν
, and so on.
Figure 6
The wavelength of the light emitted by heated metal, such as the
iron above, depends on the temperature. At room temperature, iron is gray.
When heated, it first turns red, then glowing orange.
Identify
the color of the piece of iron with the greatest kinetic energy.
Lesson 1 • Light and Quantized Energy
111
©Jack Sullivan/Alamy









