
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Applying Practice:
Wave Characteristics
HS-PS4-1.
Use mathematical representations to support a claim regarding
relationships
among
the frequency, wavelength, and speed of waves traveling in various media.
Identify Crosscutting Concepts
Create a table of the
crosscutting concepts
and fill in examples you find as you read.
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
The Atom and Unanswered Questions
After discovering three subatomic particles in the early 1900s, scientists continued their
quest to understand atomic structure and the arrangement of electrons within atoms.
Rutherford proposed that all of an atom’s positive charge and virtually all of its mass are
concentrated in a nucleus that is surrounded by fast-moving electrons. The model did not
explain how the atom’s electrons are arranged in the space around the nucleus. Nor did it
address the question of why the negatively charged electrons are not pulled into the
atom’s positively charged nucleus. Rutherford’s nuclear model did not begin to account
for the differences and similarities in chemical behavior among the various elements.
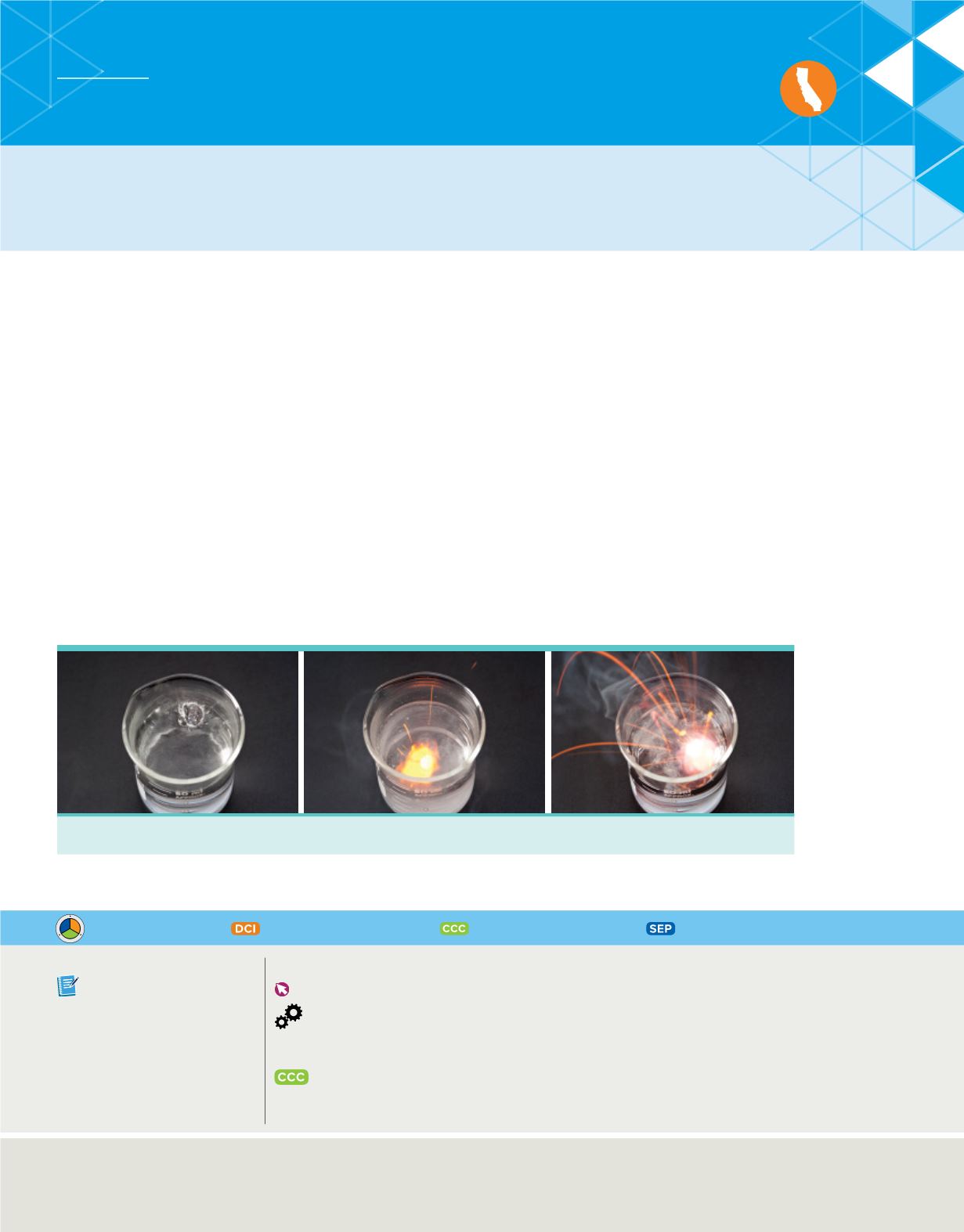
For example, consider the elements lithium, sodium, and potassium, which are found in
different periods on the periodic table but have similar chemical behaviors. All three
elements appear metallic in nature, and their atoms react vigorously with water to
liberate hydrogen gas. In fact, as shown in
Figure 1
, both sodium and potassium react
so violently that the hydrogen gas can ignite and even explode.
Sodium
Potassium
Lithium
Figure 1
Different elements can have similar reactions with water.
FOCUS QUESTION
What is light made of?
LESSON 1
LIGHT AND QUANTIZED ENERGY
106
Module 4 • Electrons in Atoms
Matt Meadows/McGraw-Hill Education









