
C04_015A
Atomizer
Source of
ionizing
radiation
Positively
charged plate
Telescope
Negatively
charged plate
(
+
)
( )
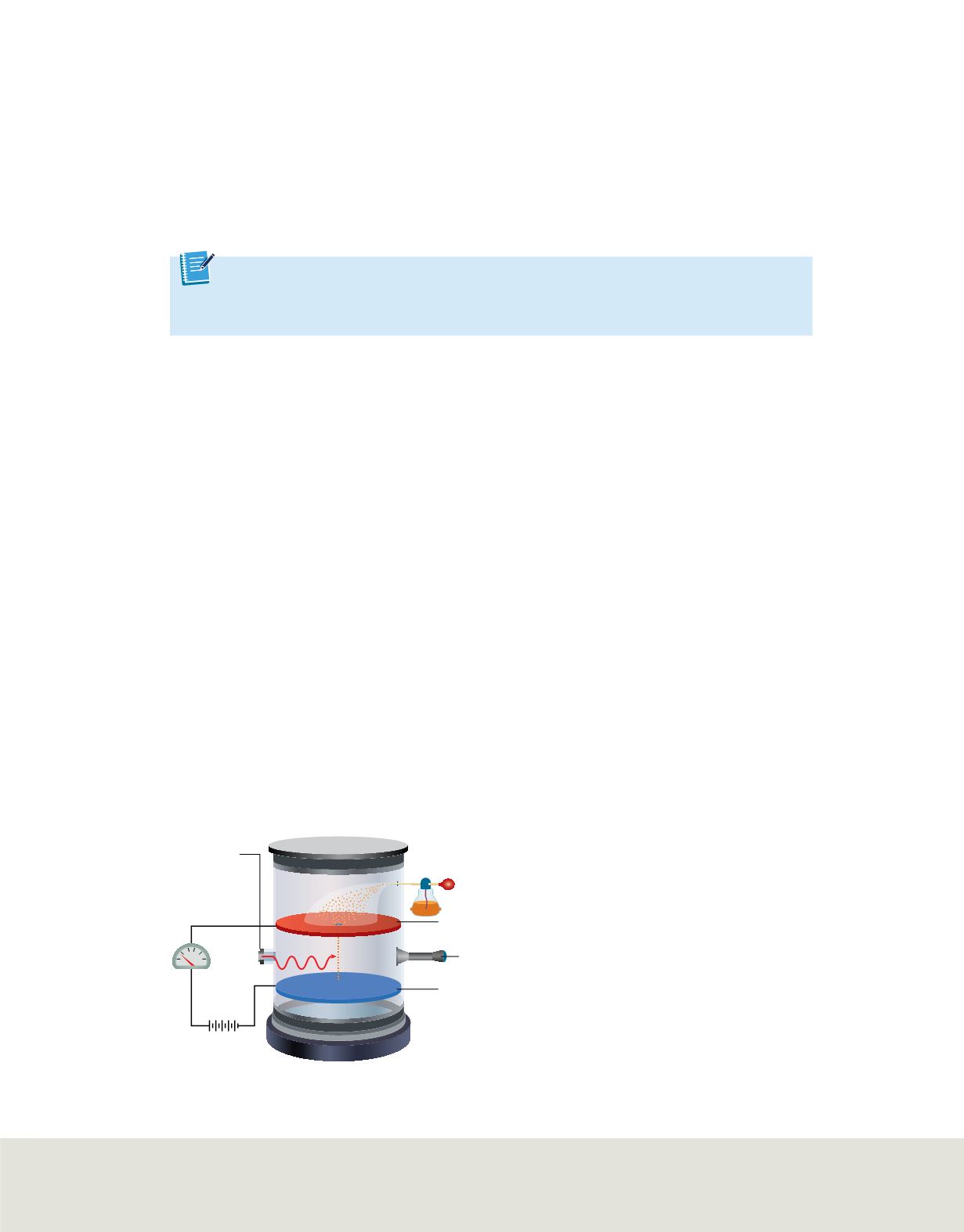
Figure 8
The motion of oil droplets within Millikan’s
apparatus depends on the charge of droplets on the
electric field. Millikan observed the droplets with the
telescope. He could make droplets fall more slowly, rise, or
pause has he varied the strength of the electric field. From
his observations, he calculated the charge on each droplet.
Thomson concluded that the mass of the charged particle was much less than that of a
hydrogen atom, the lightest known atom. The conclusion was shocking because it
meant there were particles smaller than the atom. In other words, Dalton had been
incorrect—atoms were divisible into smaller subatomic particles. Because Dalton’s
atomic theory had become so widely accepted and Thomson’s conclusion was so
revolutionary, many other scientists found it hard to accept this new discovery. But
Thomson was correct. He had identified the first subatomic particle—the electron.
He received a Nobel Prize in 1906 for this discovery.
The oil-drop experiment and the charge of an electron
The next significant
development came in the early 1910s, when the American physicist Robert Millikan
(1868–1953) determined the charge of an electron using the oil-drop apparatus shown
in
Figure 8
. In this apparatus, oil is sprayed into the chamber above the two parallel
charged plates. The top plate has a small hole through which the oil drops. X-rays
knock out electrons from the air particles between the plates and the electrons stick to
the droplets, giving them a negative charge. By varying the intensity of the electric
field, Millikan could control the rate of a droplet’s fall. He determined that the
magnitude of the charge on each drop increased in discrete amounts and determined
that the smallest common denominator was 1.602
×
10
–
19
coulombs. He identified this
number as the charge of the electron. This charge was later equated to a single unit of
negative charge noted 1
–
; in other words, a single electron carries a charge of 1
–
. So
good was Millikan’s experimental setup and technique that the charge he measured
almost one hundred years ago is within 1% of the currently accepted value.
Mass of an electron
Knowing the electron’s charge and using the known charge-to-
mass ratio, Millikan calculated the mass of an electron. The equation below shows how
small the mass of an electron is.
Mass of an electron
=
9.1
×
10
-
28
g
=
1 ____ 1840
the mass of a hydrogen atom
Get It?
Summarize
how Crookes and Thomson contributed to our understanding of the
nature of the electron.
Lesson 2 • Defining the Atom
85









