
(t)lynx/iconotec.com/Glow Images, (b)NASA
The Atom
Many experiments since Dalton’s time have proven that
atoms do exist. So what exactly is the definition of an
atom? To answer this question, consider a gold ring.
Suppose you decide to grind the ring down into a pile
of gold dust. Each fragment of gold dust still retains all
of the properties of gold. If it were possible—which it is
not without special equipment—you could continue to
divide the gold dust particles into still smaller particles.
Eventually, you would encounter a particle that could not
be divided any further and still retain the properties of
gold. This smallest particle of matter that retains the
properties of the element is called an
atom.
To get an idea of its size, consider the population of the
world, which was about 6.5
×
10
9
in 2006. By comparison, a
typical solid-copper penny contains 2.9
×
10
22
atoms, almost
five trillion times the world population! The diameter of a
single copper atom is 1.28
×
10
-
10
m. Placing 6.5
×
10
9
copper atoms side by side would result in a line of copper
atoms less than 1 m long.
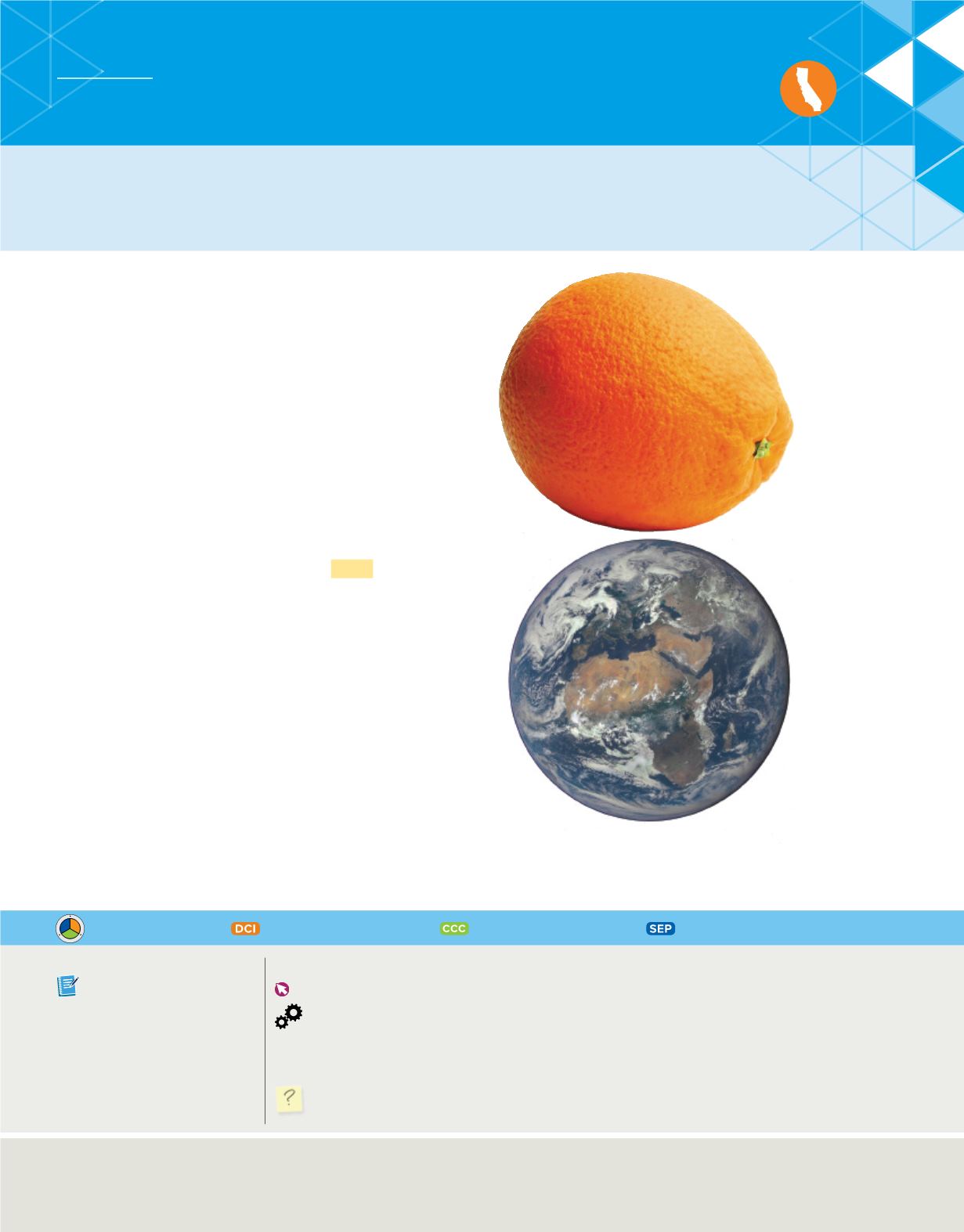
Figure 4
illustrates another way to
visualize the size of an atom. Imagine that you increase the
size of an atom to be as big as an orange. To keep the propor-
tions between the real sizes of the atom and the orange, you
would have to increase the size of the orange and make it as
big as Earth. This illustrates how small atoms are. It was
because of this that scientists could not see the atom they
were attempting to model.
Figure 4
Imagine that you could increase the size
of an atom to make it as big as an orange. At this
new scale, an orange would be as big as Earth.
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Applying Practices:
Modeling Electrostatic Forces–The Early Atom
HS-PS2-4.
Use mathematical representations of
Newton’s Law of Gravitation and
Coulomb’s Law
to describe and predict the gravitational and electrostatic forces between
objects.
Revisit the Encounter the Phenomenon Question
What information from this lesson can help you answer the module question?
FOCUS QUESTION
What does an atom look like?
LESSON 2
DEFINING THE ATOM
82
Module 3 • The Structure of the Atom









