
C04_018A
Electrons
Alpha particle paths
Evenly distributed
positive charge
- -
-
-
-
- -
- -
-
-
-
-
C04_019A
Gold foil
Beam of
alpha
particles
Most alpha particles
pass through the
foil with little or
no deflection
Lead block containing
an alpha-particle-
emitting source
Zinc-sulfide-
coated screen
Alpha particle
deflected at a
small angle
Alpha particle
deflected at a
large angle
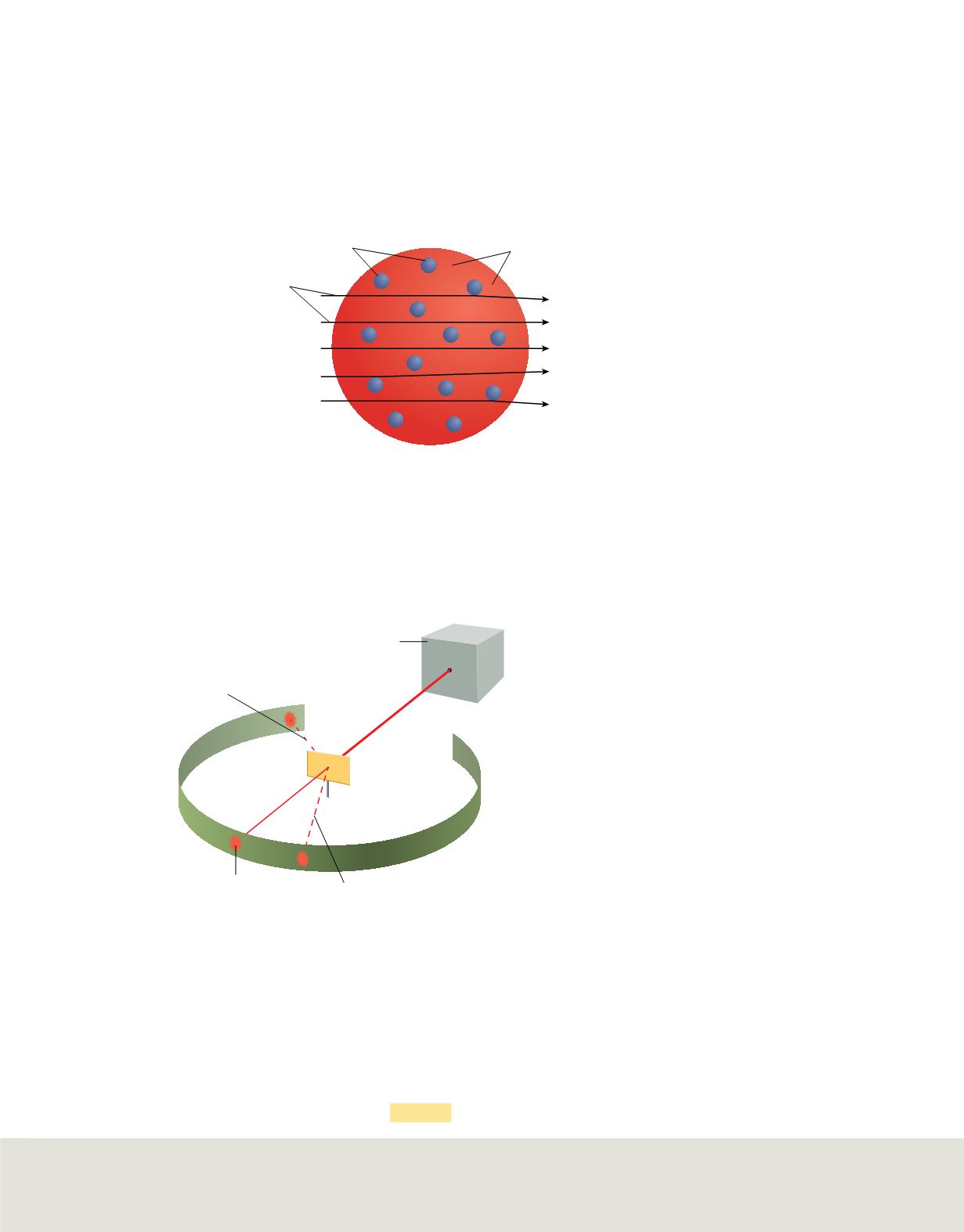
Rutherford was aware of Thomson’s plum pudding model of the atom. He expected the
paths of the massive and fast-moving alpha particles to be only slightly altered by a
collision with an electron. And because the positive charge within the gold atoms was
thought to be uniformly distributed, he thought it would not alter the paths of the alpha
particles, either.
Figure 10
shows the results Rutherford expected from the experiment.
Figure 10
Based on Thomson’s model,
Rutherford expected the alpha particles to
pass through the gold atoms.
The actual results observed by Rutherford and his colleagues are shown in
Figure 11
.
Some alpha particles were deflected at various angles. Several particles were deflected
straight back toward the source. Rutherford likened the results to firing a large artillery
shell at a sheet of paper and the shell coming back at the cannon.
Figure 11
During Rutherford’s experiment, a beam of
alpha particles bombarded a thin gold foil. Most of the
alpha particles went through the gold foil. However, a few
of them bounced back, some at large angles.
Rutherford’s model of the atom
Rutherford concluded that the plum pudding
model was incorrect because it could not explain the results of the gold foil experiment.
Considering the properties of the alpha particles and the electrons, and the frequency
of the deflections, he calculated that an atom consisted mostly of empty space through
which the electrons move. He also concluded that almost all of the atom’s positive
charge and almost all of its mass were contained in a tiny, dense region in the center of
the atom, which he called the
nucleus.
Lesson 2 • Defining the Atom
87









