
C04_020A
Nucleus
Alpha particle
paths
Electrons
- -
-
-
-
- -
-
-
+ -
-
-
-
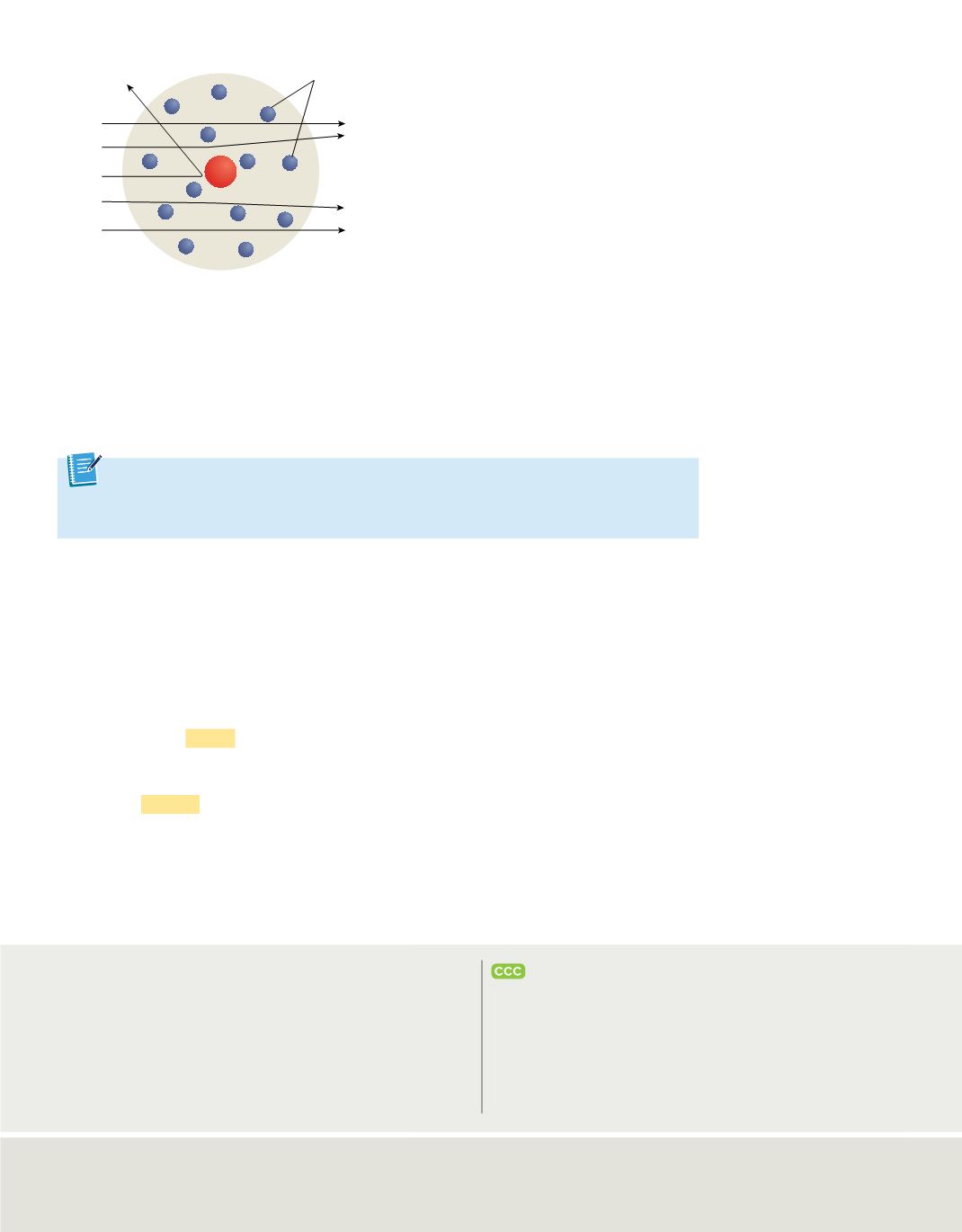
Figure 12
In Rutherford’s nuclear model, the atom is
composed of a dense, positively charged nucleus
that is surrounded by negative electrons. Alpha
particles passing far from the nucleus are only
slightly deflected, if at all. Those passing closer to
the nucleus are deflected more. Alpha particles
directly approaching the nucleus are deflected at
large angles.
The negatively charged electrons are held within the atom by their attraction to the
positively charged nucleus. Rutherford’s nuclear atomic model is shown in
Figure 12
.
Because the nucleus occupies such a small space and contains most of an atom’s mass,
it is incredibly dense. If a nucleus were the size of the dot in an exclamation point, its
mass would be amost as much as 70 automobiles! The volume of space through which
the electrons move is huge compared to the volume of the nucleus. If an atom had a
diameter of two football fields, the nucleus would be the size of a nickel.
The repulsive force produced between the positive nucleus and the positive alpha parti-
cles causes the deflections.
Figure 12
illustrates how Rutherford’s nuclear atomic model
explained the results of the gold foil experiment. The nuclear model also explains the
neutral nature of matter: the positive charge of the nucleus balances the negative charge
of the electrons. However, the model still could not account for all of the atom’s mass.
The proton and the neutron
By 1920, Rutherford concluded that the nucleus contained positively charged particles
called protons. A
proton
is a subatomic particle carrying a charge equal to but opposite
that of an electron. In 1932, Rutherford’s coworker, James Chadwick (1891–1974),
showed that the nucleus also contained another subatomic neutral particle, called the
neutron. A
neutron
is a subatomic particle that has a mass nearly equal to that of a
proton, but carries no electric charge.
Completing the model of the atom
All atoms have a charged substructure
consisting of a small, dense nucleus, which is made of protons and neutrons, surrounded
by one or more electrons.
Figure 13,
on the next page, summarizes the history and
development of modern atomic theory.
Get It?
Describe
how the photograph at the beginning of the module is related to
Rutherford’s ideas about what atoms are made of.
SCIENCE USAGE V. COMMON USAGE
neutral
Science usage:
to have no electric charge
Neutrons have a charge of zero. They are neutral particles.
Common usage:
not engaged in either side
Switzerland remained neutral during World War II.
CROSSCUTTING CONCEPTS
System and System Models
Review the system
models used by Crookes, Thomson, Millikan,
Rutherford, and Chadwick that led to the
development of the completed model of the atom.
Develop a time line describing their model
simulations, instruments, and contributions to
atomic theory.
88
Module 3 • The Structure of the Atom









