
Science Source
Table 2
Dalton’s Atomic Theory
Scientist
Ideas
Dalton
(1766–1844)
• Matter is composed of extremely small particles called atoms.
• Atoms are indivisible and indestructible.
• Atoms of a given element are identical in size, mass, and chemical properties.
• Atoms of a specific element are different from those of another element.
• Different atoms combine in simple whole-number ratios to form compounds.
• In a chemical reaction, atoms are separated, combined, or rearranged.
John Dalton
Although the concept of the atom was revived in the eighteenth century, it took another
hundred years before significant progress was made. The work done in the nineteenth
century by John Dalton (1766–1844), a schoolteacher in England, marks the beginning
of the development of modern atomic theory. Dalton revived and revised Democritus’s
ideas based on the results of scientific research he conducted. In many ways, Democri-
tus’s and Dalton’s ideas are similar.
Thanks to advancements in science since Democritus’s day, Dalton was able to perform
experiments that allowed him to identify patterns that refined previous understandings
and developed new explanations that were supported by his research. He studied
numerous chemical reactions, making careful observations and measurements along
the way. He was able to determine the mass ratios of the elements involved in those
reactions. The mass ratio describes how many atoms of each element make up a com-
pound. The results of his research are known as
Dalton’s atomic theory,
which he
proposed in 1803. The main points of his theory are summarized in
Table 2
. Dalton
published his ideas in a book, an extract of which is shown in
Figure 2
.
Get It?
Explain
how Dalton built upon the ideas that Democritus had about matter.
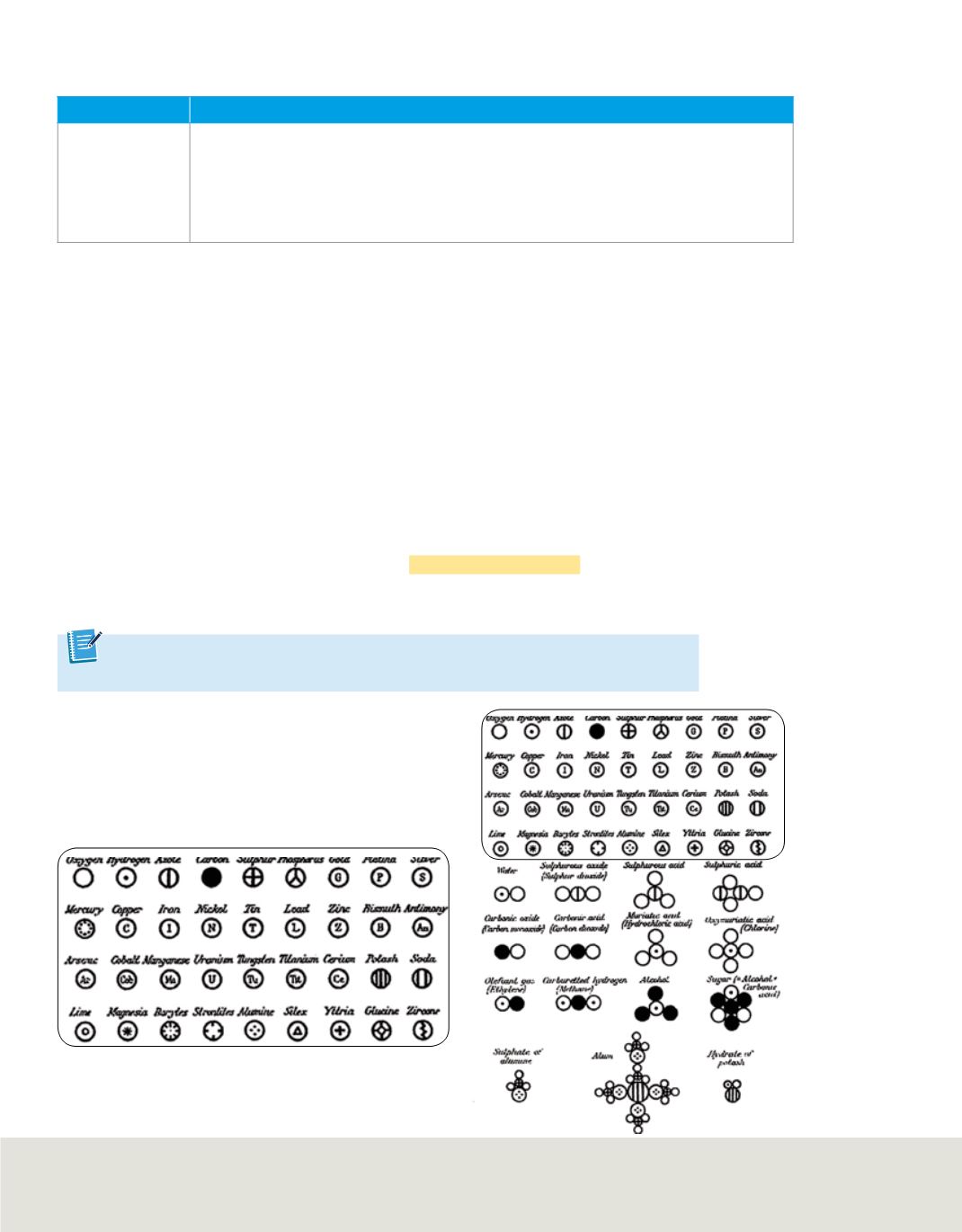
Figure 2
In his book,
A New System of Chemical
Philosophy
, John Dalton presented his symbols
for the elements known at that time
and their possible combinations.
80
Module 3 • The Structure of the Atom









