
C04_023A
Hydrogen
1.008
Atomic
number
Average
atomic mass
Chemical
symbol
Chemical
name
1
H
Atomic Number
There are more than 110 different elements on the periodic table of the elements. What
makes an atom of one element different from an atom of another element? After Ruther-
ford’s gold foil experiment, the English scientist Henry Moseley (1887–1915) discovered that
an element contains a unique positive charge in its nucleus. Thus, the number of protons in
an atom identifies it as an atom of a particular element. The number of protons in an atom
is referred to as the
atomic number.
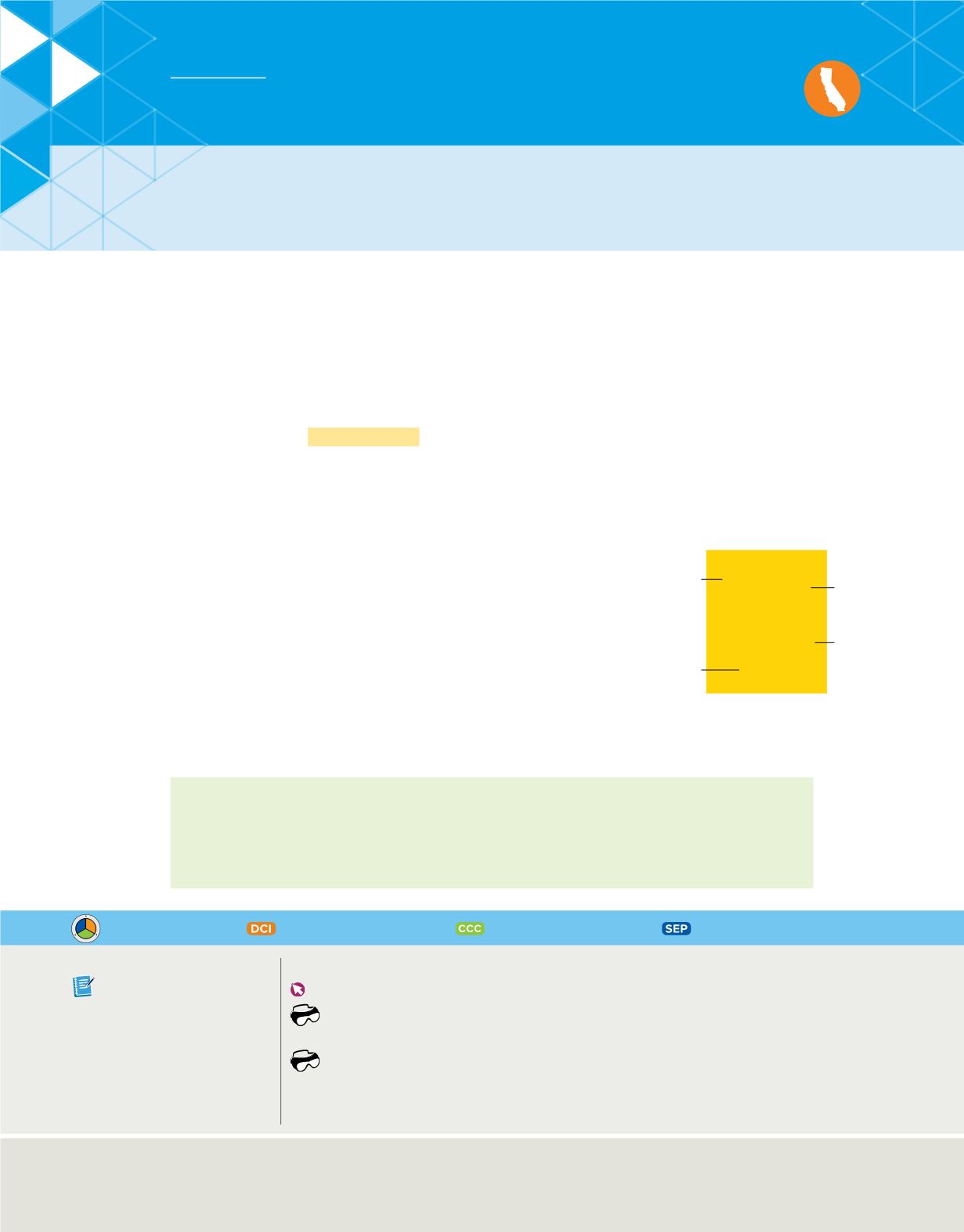
Hydrogen’s square on the periodic table is shown in
Figure 15
. The number 1 to the left of the symbol is the atomic number. Moving across the
periodic table to the right, you will next come to helium (He). It has two protons in its
nucleus; thus, it has an atomic number of 2. The next row begins with lithium (Li), atomic
number 3, and so on. The periodic table is organized left-to-right and top-to-bottom by
increasing atomic number. The pattern of repeating order is
called periodicity, which accounts for the name given to this
table.
Elements are listed in their neutral state on the periodic
table. Therefore, once you know the atomic number of an
element, you know the number of protons and electrons it
contains. Lithium, with an atomic number of 3, has three
protons and three electrons. Thus, the periodicity of the
elements in the periodic table provides a clue as to how the
structure of each element differs.
Figure 15
In the periodic table, each element is
represented by its chemical name, atomic number,
chemical symbol, and average atomic mass.
Atomic number
atomic number
=
number of protons
=
number of electrons
The atomic number of an atom equals its number of protons and its number of electrons.
LESSON 3
HOW ATOMS DIFFER
FOCUS QUESTION
Are all atoms identical?
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Virtual Investigation:
Decoding the Periodic Table
Analyze and interpret data
to identify the
patterns
in
the structure and properties of matter.
Quick Investigation:
Model Isotopes
Plan and carry out an investigation
to create a
model
of
stable forms of matter.
Lesson 3 • How Atoms Differ
91









