
WATER
FIRE
EARTH
AIR
Dry
Cold
Hot
Wet
(t)Weeping Willow Photography/Moment/Getty Images, (l b)McGraw-Hill Education, (r)Tinke Hamming/Ingram Publishing
The Roots of Atomic Theory
Science as we know it today did not exist several thousand years ago. No one knew what
a controlled experiment was, and there were few tools for scientific exploration. In this
setting, the power of the mind and intellectual thought were considered the primary
avenues to the truth. Curiosity sparked the interest of scholarly thinkers known as
philosophers who considered the many mysteries of life. As they speculated about the
nature of matter, many of the philosophers formulated explanations based on their own
life experiences.
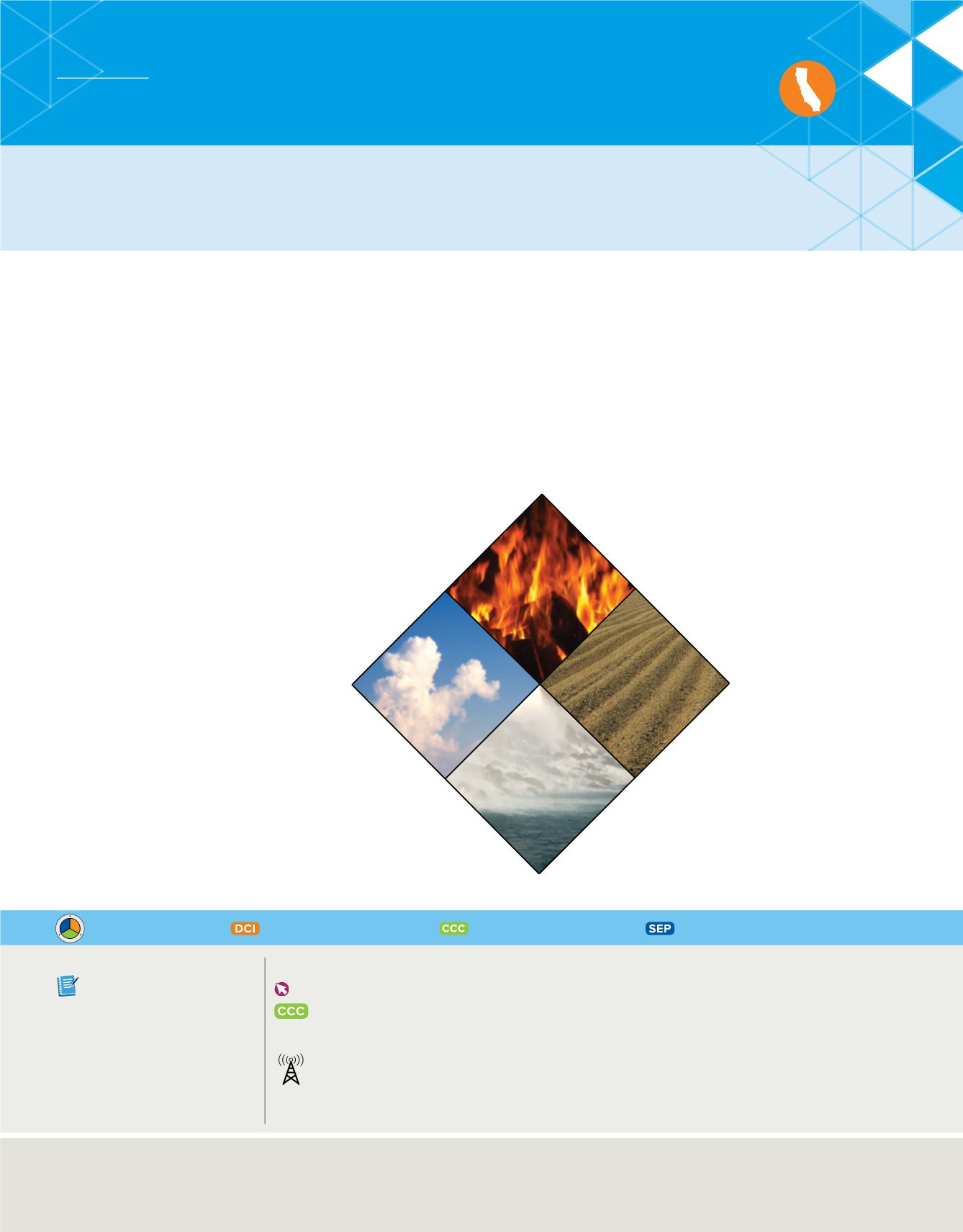
Many of these philosphers con-
cluded that matter was composed
of things such as earth, water, air,
and fire, as shown in
Figure 1
. It
was also commonly accepted that
matter could be endlessly divided
into smaller and smaller pieces.
While these early ideas were
creative, there was no method
available to test their validity, and
so these ideas could not be shown
to be correct.
Figure 1
Many Greek philosophers thought
that matter was composed of four elements:
earth, air, water, and fire. They also
associated properties with each element.
The pairing of opposite properties such as
hot and cold, and wet and dry, mirrored the
symmetry they observed in nature. These
early ideas were non-scientific.
FOCUS QUESTION
How has our understanding of matter changed over time?
LESSON 1
EARLY IDEAS ABOUT MATTER
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Identify Crosscutting Concepts
Create a table of the
crosscutting concepts
and fill in examples you find as you read.
Review the News
Obtain information
from a current news story about
chemical processes, their rates, and
whether or not energy is conserved.
Evaluate
your source and
communicate
your findings
to the class.
78
Module 3 • The Structure of the Atom









