
Table 1
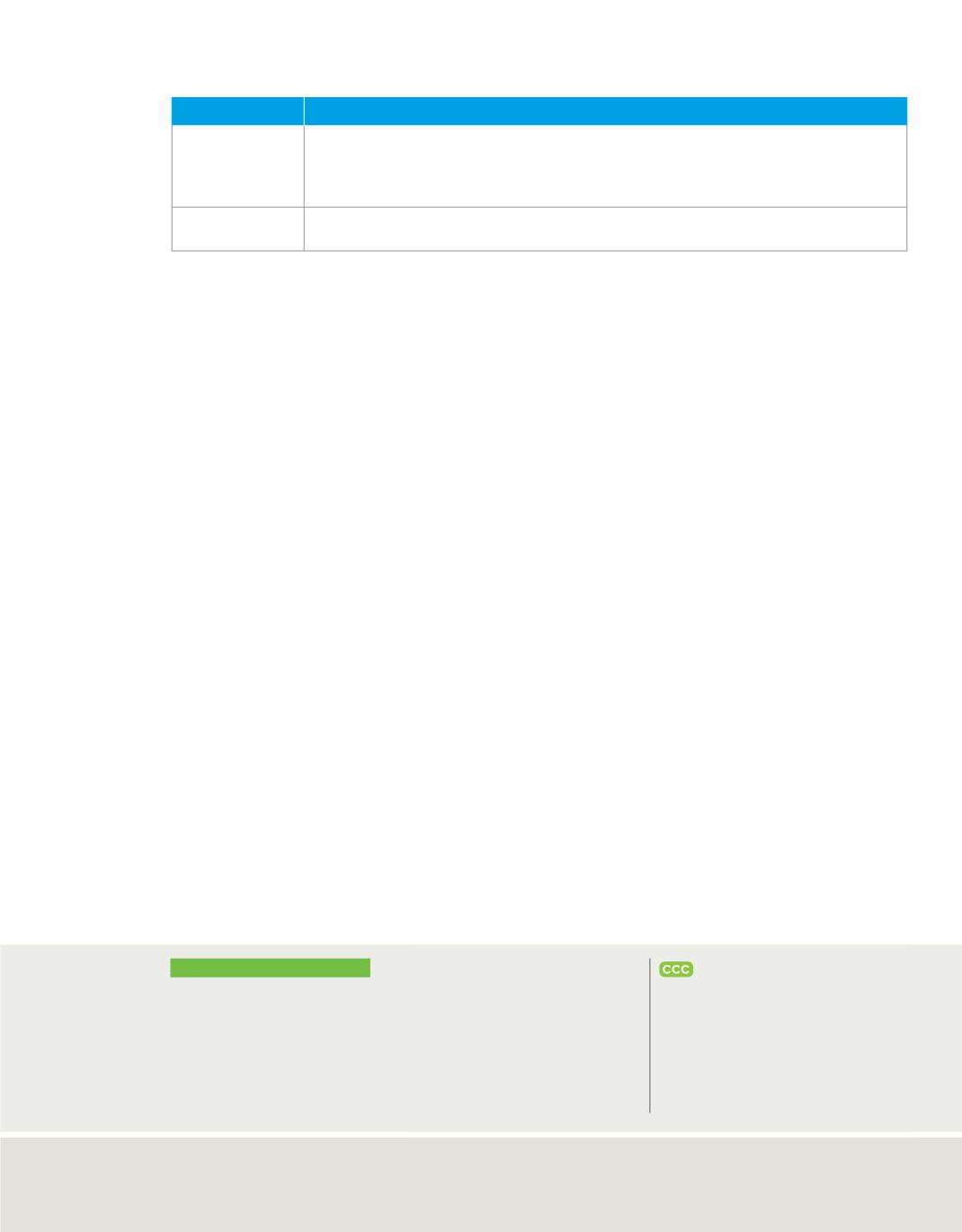
Ancient Greek Ideas About Matter
Philosopher
Ideas
Democritus
(460–370 b.c.)
• Matter is composed of atoms, which move through empty space.
• Atoms are solid, homogeneous, indestructible, and indivisible.
• Different kinds of atoms have different sizes and shapes.
• Size, shape, and movement of atoms determine the properties of matter.
Aristotle
(384–322 b.c.)
• Empty space cannot exist.
• Matter is made of earth, fire, and water.
Democritus
The Greek philosopher Democritus (460–370
b
.
c
.
) was the first person to propose the
idea that matter was not infinitely divisible. He believed matter was made up of tiny
particles called atomos, from which the English word atom is derived. Democritus
believed that
atoms
could not be created, destroyed, or further divided. Democritus and
a summary of his ideas are shown in
Table 1
.
While a number of Democritus’s ideas do not agree with modern atomic theory, his
belief in the existence of atoms was amazingly ahead of his time. However his ideas
were met with criticism from other philosophers who asked, “What holds the atoms
together?” Democritus could not answer this question.
Aristotle
Other criticisms came from Aristotle (384–322
b
.
c
.
), one of the most influential Greek
philosophers. He rejected the notion of atoms because it did not agree with his own
ideas about nature. One of Aristotle’s major criticisms concerned the idea that atoms
moved through empty space. He did not believe that empty space could exist. His ideas
are also presented in
Table 1
. Because Aristotle was one of the most influential philoso-
phers of his time, Democritus’s atomic theory was eventually rejected.
In fairness to Democritus, it was impossible for him or anyone else of his time to deter-
mine what held atoms together. More than two thousand years would pass before
scientists would know the answer. However, it is important to realize that Democritus’s
ideas were just that—ideas, not science. Without the ability to conduct controlled
experiments, Democritus could not identify patterns, test the validity of his ideas, and
collect evidence to support his hypotheses.
Unfortunately for the advancement of science, Aristotle was able to gain wide accep-
tance for his ideas on nature—ideas that denied the existence of atoms. Incredibly, the
influence of Aristotle was so great and the development of science so primitive that his
denial of the existence of atoms went largely unchallenged for two thousand years!
STEM CAREER Connection
Chemistry Professor
If you like spending time with people and have a passion for chemistry and
research, professors spend their time instructing students at a wide variety of
colleges, universities, and community colleges. In addition, they conduct research
and publish scholarly papers and books. You will need a master’s degree or Ph.D.
depending on the institution’s requirements.
CROSSCUTTING CONCEPTS
Patterns
Examine the contributions of
Democritus, Aristotle, and Dalton. In groups
of two, make a poster that compares and
contrasts any methods that were used or
patterns that were identified that provided
evidence in explaining the nature of matter
and the atom.
Lesson 1 • Early Ideas About Matter
79









