
Summary
• The arrangement of electrons
in an atom is called the atom’s
electron configuration.
• Electron configurations are
defined by the aufbau princi-
ple, the Pauli exclusion princi-
ple, and Hund’s rule.
• An element’s valence electrons
determine the chemical proper-
ties of the element.
• Electron configurations can be
represented using orbital
diagrams, electron configura-
tion notation, and electron-dot
structures.
Demonstrate Understanding
27.
Apply
the Pauli exclusion principle, the aufbau
principle, and Hund’s rule to write the electron
configuration and draw the orbital diagram for each of
the following elements.
a.
silicon b. fluorine c. calcium d. krypton
28.
Define
valence electron
.
29.
Illustrate
and describe the sequence in which ten
electrons occupy the five orbitals related to an atom’s
d sublevel.
30.
Extend
the aufbau sequence through an element that
has not yet been identified, but whose atoms would
completely fill 7p orbitals. How many electrons would
such an atom have? Write its electron configuration using
noble-gas notation for the previous noble gas, radon.
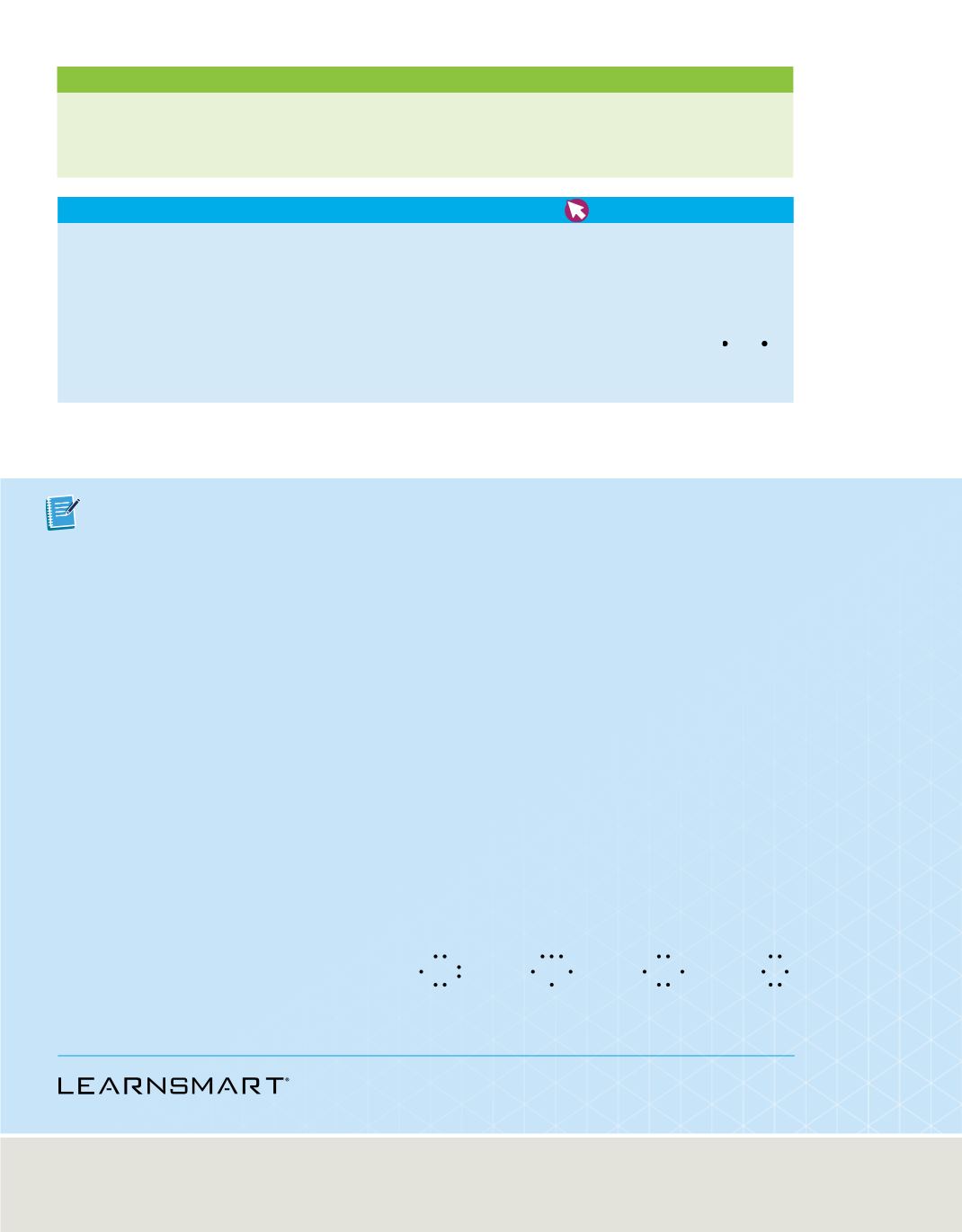
31.
Interpret Scientific Illustrations
Which is the correct
electron-dot structure for an atom of selenium? Explain.
a.
b.
c.
d.
Check Your Progress
PRACTICE
Problems
24.
Draw electron-dot structures for atoms of the following elements.
a.
magnesium
b.
thallium
c.
xenon
25.
An atom of an element has a total of 13 electrons. What is the element, and how many electrons
are shown in its electron-dot structure?
26. CHALLENGE
This element exists in the solid state at room temperature and at normal
atmospheric pressure and is found in emerald gemstones. It is known to be one of the
following elements: carbon, germanium, sulfur, cesium, beryllium, or argon. Identify the
element based on the electron-dot structure at right.
C05_038A
X
EXAMPLE
Problem 3 (continued)
3
EVALUATE THE ANSWER
The correct symbol for tin (Sn) has been used, and the rules for drawing electron-dot structures
have been correctly applied.
C05_039A
Se
Se
Se
S
Go online to follow your personalized learning path to review, practice,
and reinforce your understanding.
ADDITIONAL PRACTICE
132
Module 4 • Electrons in Atoms









