
ADDITIONAL PRACTICE
PRACTICE
Problems
19.
Write ground-state electron configurations for the following elements.
a.
bromine (Br)
c.
antimony (Sb)
e.
terbium (Tb)
b.
strontium (Sr)
d.
rhenium (Re)
f.
titanium (Ti)
20.
A chlorine atom in its ground state has a total of seven electrons in orbitals related to the atom’s
third energy level. How many of the seven electrons occupy p orbitals? How many of the 17
electrons in a chlorine atom occupy p orbitals?
21.
When a sulfur atom reacts with other atoms, electrons in the atom’s third energy level are involved.
How many such electrons does a sulfur atom have?
22.
An element has the ground-state electron configuration [Kr]5s
2
4d
10
5p
1
. It is part of some
semiconductors and used in various alloys. What element is it?
23. CHALLENGE
In its ground state, an atom of an element has two electrons in all orbitals related
to the atom’s highest energy level for which
n
=
6. Using noble-gas notation, write the electron
configuration for this element, and identify the element.
Exceptions to predicted configurations
You can use the aufbau diagram to write correct ground-state electron configurations
for all elements up to and including vanadium, atomic number 23. However, if you were
to proceed in this manner, your configurations for chromium, [Ar]4s
2
3d
4
, and copper,
[Ar]4s
2
3d
9
, would be incorrect. The correct configurations for these two elements are
[Ar]4s
1
3d
5
for chromium and [Ar]4s
1
3d
10
for copper. The electron configurations for
these two elements, as well as those of several other elements, illustrate the increased
stability of half-filled and filled sets of s and d orbitals.
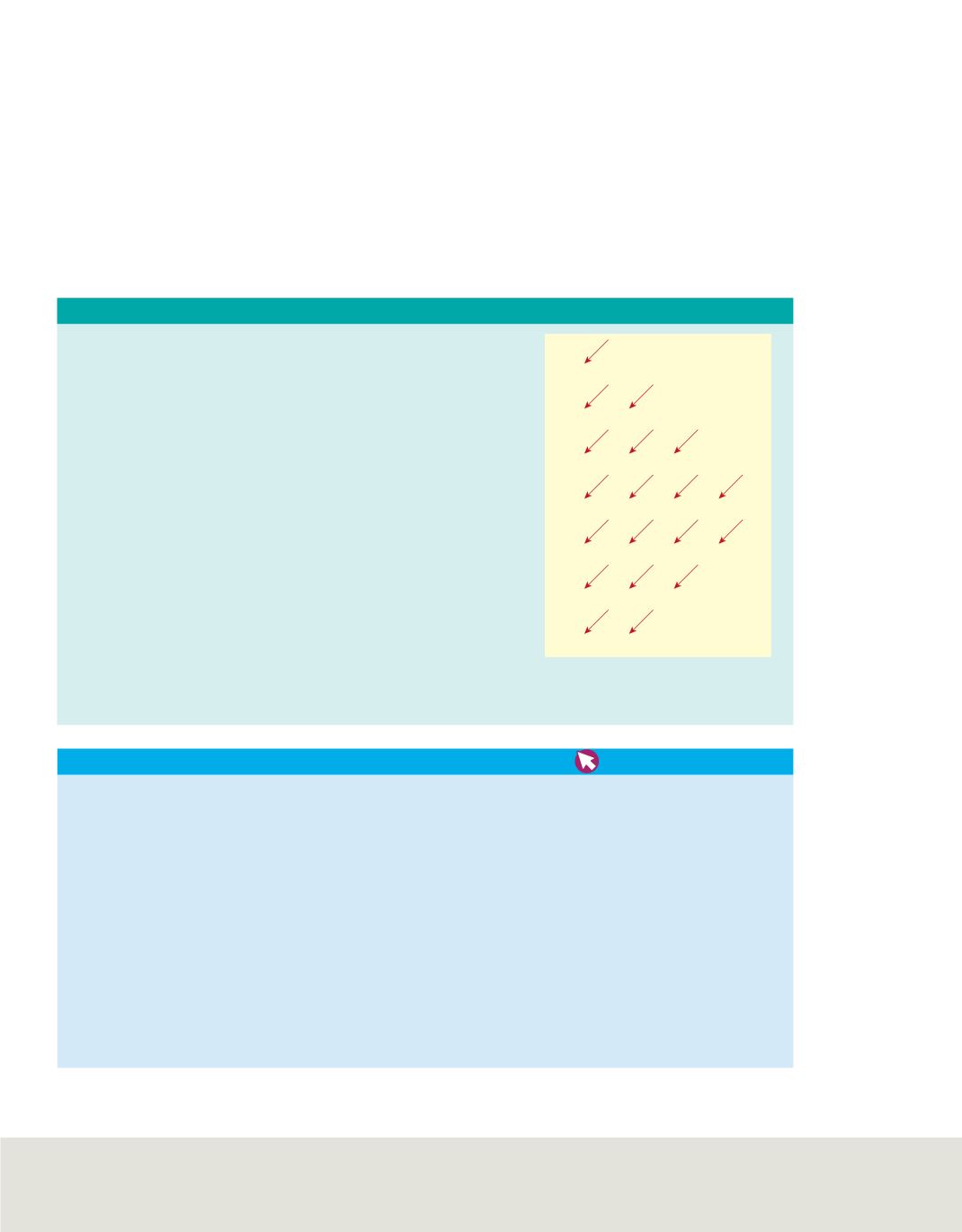
PROBLEM-SOLVING STRATEGY
The sublevel diagram shows the order
in which the orbitals are usually filled.
C05_035A
1s
2s
3s
4s
5s
6s
7s
2p
3p
4p
5p
6p
7p
3d
4d
5d
6d
4f
5f
Filling Atomic Orbitals
By drawing a sublevel diagram and following the arrows, you can
write the ground-state electron configuration for any element.
1.
Sketch the sublevel diagram on a blank piece of paper.
2.
Determine the number of electrons in one atom of the element for
which you are writing the electron configuration. The number of
electrons in a neutral atom equals the element’s atomic number.
3.
Starting with 1s, write the aufbau sequence of atomic orbitals
by following the diagonal arrows from the top of the sublevel
diagram to the bottom. When you complete one line of arrows,
move to the right, to the beginning of the next line of arrows.
As you proceed, add superscripts indicating the numbers of
electrons in each set of atomic orbitals. Continue only until
you have sufficient atomic orbitals to accommodate the total
number of electrons in one atom of the element.
4.
Apply noble-gas notation.
Apply the Strategy
Write
the ground-state electron configuration for zirconium.
I I
I
130
Module 4 • Electrons in Atoms









