
EXAMPLE
Problem 2
USE ATOMIC NUMBER AND MASS NUMBER
A chemistry laboratory has analyzed the
composition of isotopes of several elements. The composition data is given in the table below.
Determine the number of protons, electrons, and neutrons in the isotope of neon. Name the
isotope and give its symbol.
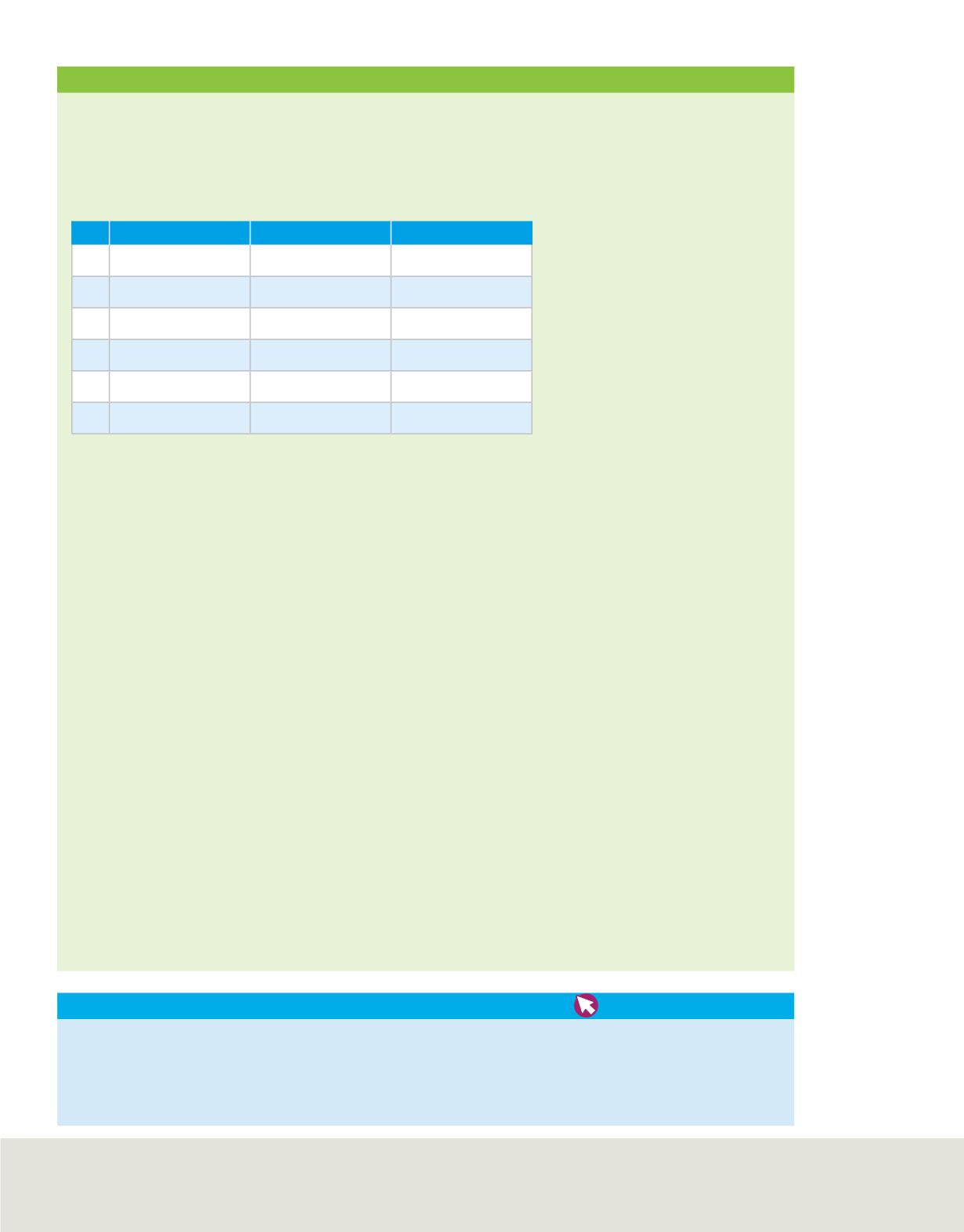
Isotope Composition Data
Element
Atomic Number
Mass Number
a.
Neon
10
22
b.
Calcium
20
46
c.
Oxygen
8
17
d.
Iron
26
57
e.
Zinc
30
64
f.
Mercury
80
204
1
ANALYZE THE PROBLEM
You are given some data for neon in the table. The symbol for neon can be found on the
periodic table. From the atomic number, the number of protons and electrons in the isotope are
known. The number of neutrons in the isotope can be found by subtracting the atomic number
from the mass number.
Known
Unknown
element: neon
number of protons
(
N
p
)
, electrons
(
N
e
)
, and neutrons
(
N
n
)
=
?
atomic number
=
10
name
of isotope
=
?
mass number
=
22
symbol
for isotope
=
?
2
SOLVE FOR THE UNKNOWN
number of protons
=
atomic number
=
10
number of electrons
=
atomic number
=
10
number of neutrons
=
mass number
-
atomic number
N
n
=
22
-
10
=
12
The
name
of the isotope is
neon-22.
The
symbol
for the isotope is
22
10
Ne
.
3
EVALUATE THE ANSWER
The relationships among number of electrons, protons, and neutrons have been applied
correctly. The isotope’s name and symbol are in the correct format. Refer to the Elements
Handbook to learn more about neon.
Apply the atomic number relationship.
Use the atomic number and the mass number
to calculate the number of neutrons.
Substitute mass number
=
22 and atomic
number
=
10
Use the element name and mass number to write the
isotope’s name.
Use the chemical symbol, mass number, and atomic number
to write out the isotope in symbolic notation form.
PRACTICE
Problems
15.
Determine the number of protons, electrons, and neutrons for isotopes
b.—f.
in the table above.
Name each isotope, and write its symbol.
16. CHALLENGE
An atom has a mass number of 55. Its number of neutrons is the sum of its atomic
number and five. How many protons, neutrons, and electrons does this atom have? What is the
identity of this atom?
ADDITIONAL PRACTICE
94
Module 3 • The Structure of the Atom









