
C06_023A
1
1
2
2
3
3
4
4
5
5
6
6
7
7
2
3
4
5
6
8 9 10 11
12
13
14
15
16
17
F
3.98
Cl
3.16
Br
2.96
I
2.66
At
2.2
Zn
1.65
Cd
1.69
Al
1.61
V
1.63
Cr
1.66
Mn
1.55
Fe
1.83
Co
1.88
Ni
1.91
Ag
1.93
H
2.20
Li
0.98
Be
1.57
Na
0.93
Mg
1.31
K
0.82
Ca
1.00
Sc
1.36
Ti
1.54
Rb
0.82
Cs
0.79
Fr
0.70
Ra
0.90
Ac
1.1
Ba
0.89
La
1.1
Sr
0.95
Y
1.22
Zr
1.33
Hf
1.3
Nb
1.6
Ta
1.5
Mo
2.16
W
1.7
Tc
2.10
Ru
2.2
Re
1.9
Rh
2.28
Cu
1.90
Pd
2.20
Os
2.2
Ir
2.2
Pt
2.2
Hg
1.9
Au
2.4
Tl
1.8
In
1.78
Ga
1.81
B
2.04
C
2.55
N
3.04
O
3.44
S
2.58
P
2.19
Si
1.90
Ge
2.01
As
2.18
Sb
2.05
Sn
1.96
Se
2.55
Te
2.1
Po
2.0
Bi
1.9
Pb
1.8
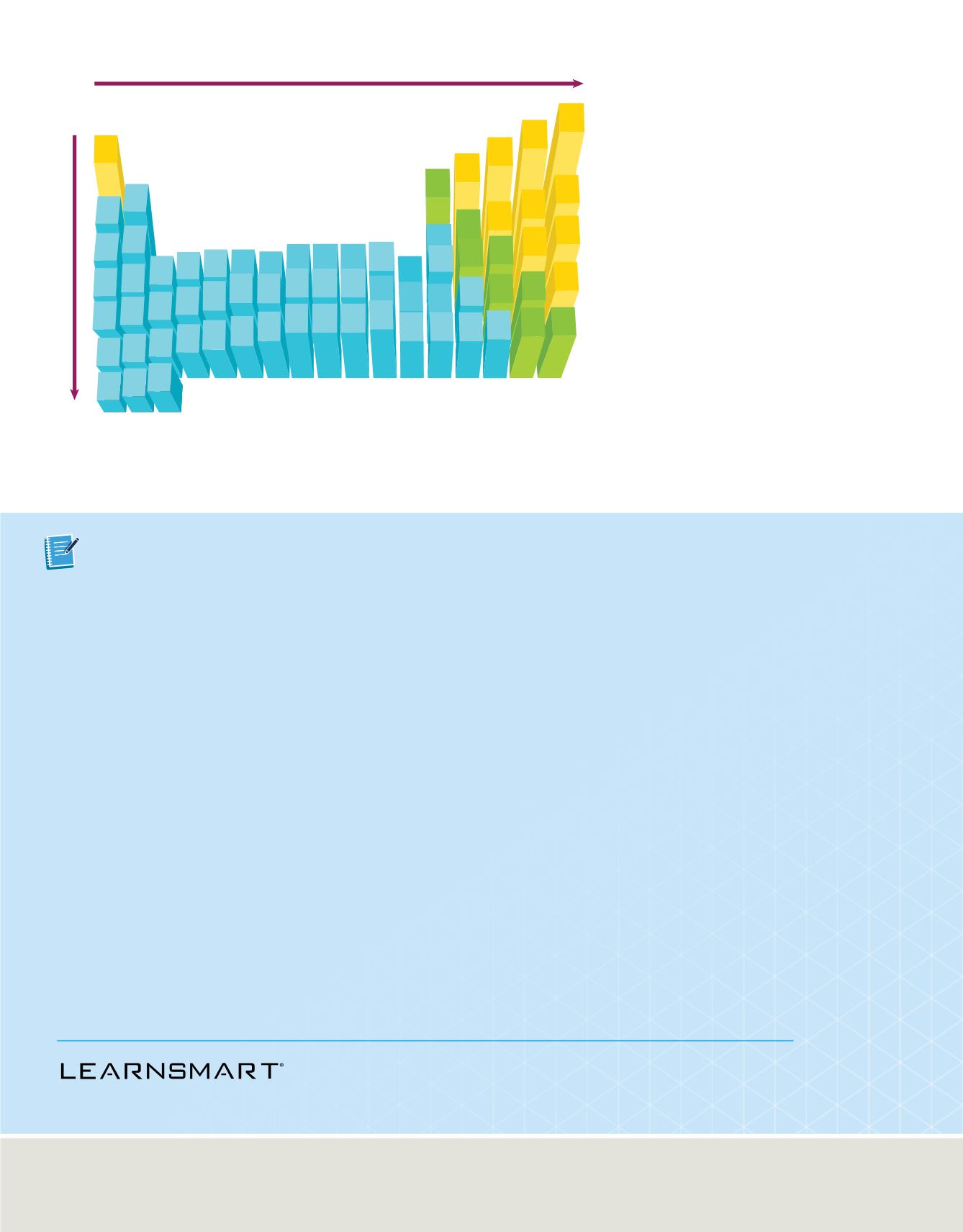
Increasing electronegativity
Electronegativity Values in Paulings
Decreasing electronegativity
Figure 19
The electronegativ-
ity values for most of the
elements are shown. The
values are given in Paulings, a
unit named after American
scientist Linus Pauling
(1901–1994).
Infer
why electronegativity
values are not listed for the
noble gases.
Go online to follow your personalized learning path to review, practice,
and reinforce your understanding.
Check Your Progress
Summary
• Atomic and ionic radii decrease
from left to right across a
period, and increase as you
move down a group.
• Ionization energies generally
increase from left to right
across a period, and decrease
down a group.
• The octet rule states that atoms
gain, lose, or share electrons to
acquire a full set of eight
valence electrons.
• Electronegativity generally
increases from left to right
across a period, and decreases
down a group.
Demonstrate Understanding
20.
Explain
how the period and group trends in atomic
radii are related to electron configuration.
2 1 .
Indicate
whether fluorine or bromine has a larger
value for each of the following properties.
a.
electronegativity c. atomic radius
b.
ionic radius
d. ionization energy
22.
Explain
why it takes more energy to remove the
second electron from a lithium atom than it does to
remove the fourth electron from a carbon atom.
23.
Calculate
Determine the differences in electronega-
tivity, ionic radius, atomic radius, and first ionization
energy for oxygen and beryllium.
24.
Make and Use Graphs
Graph the atomic radii of the
representative elements in periods 2, 3, and 4 versus
their atomic numbers. Connect the points of elements
in each period, so that there are three separate
curves on the graph. Summarize the trends in atomic
radii shown on your graph. Explain.
160
Module 5 • The Periodic Table and Periodic Law









