
C06_013A
H
37
He
31
Li
152
Na
186
K
227
Rb
248
Cs
265
Be
112
Mg
160
Ca
197
Sr
215
Ba
222
B
85
Al
143
Ga
135
n
167
Tl
170
C
77
Si
118
Ge
122
Sn
140
Pb
146
N
75
P
110
As
120
Sb
140
Bi
150
O
73
S
103
Se
119
Te
142
Po
168
F
72
Cl
100
Br
114
133
At
140
Ne
71
Ar
98
Kr
112
Xe
131
Rn
140
1
1
2
3
4
5
6
2
13
14
15
16
17
18
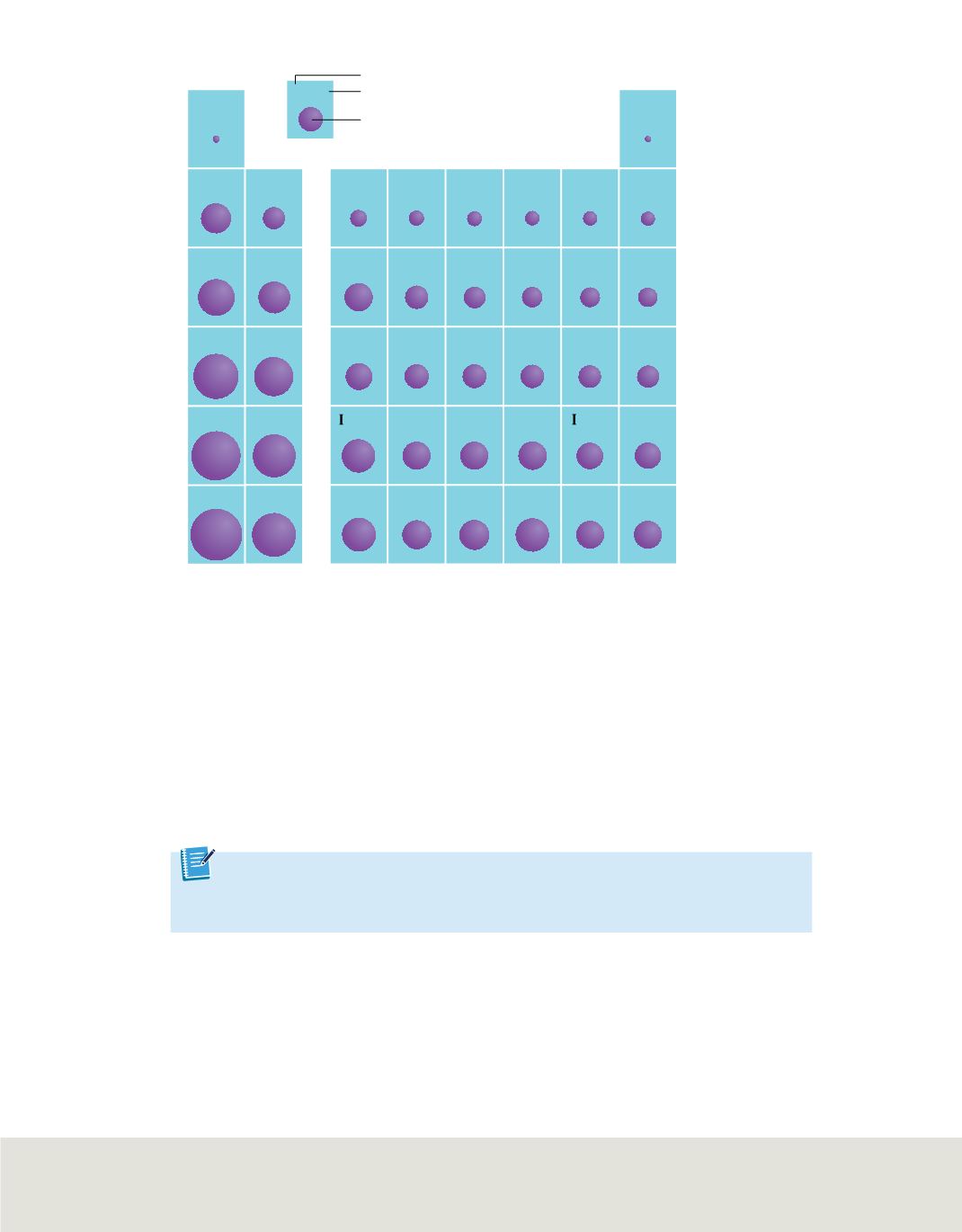
Chemical symbol
Atomic radius
Relative size
K
227
Figure 12
The atomic radii of the
representative elements, given in
picometers (10
–
12
m), vary as you
move from left to right within a
period and down a group.
Infer
why the atomic radii increase
as you move down a group.
Get It?
Discuss
how the fact that the principal energy level remains the same within a
period explains the decrease in the atomic radii across a period.
Trends within periods
In general, there is a decrease in atomic radii as you move from left to right across
a period. This trend is illustrated in
Figure 12
. It is caused by the increasing positive
charge in the nucleus and the fact that the principal energy level within a period
remains the same. Each successive element has one additional proton and electron,
and each additional electron is added to orbitals corresponding to the same princi-
pal energy level. Moving across a period, no additional electrons come between the
valence electrons and the nucleus. Thus, the valence electrons are not shielded from
the increased nuclear charge, which pulls the outermost electrons closer to the
nucleus.
Trends within groups
Atomic radii generally increase as you move down a group on the periodic table.
The nuclear charge increases, and electrons are added to orbitals corresponding to
successively higher principal energy levels. However, the increased nuclear charge
does not pull the outer electrons toward the nucleus to make the atom smaller as
you might expect. Why does the increased nuclear charge not make the atom
smaller?
Lesson 3 • Periodic Trends
153









