
C06_016A
186 pm
102 pm
Sodium atom (Na)
[Ne]3s
1
Sodium ion (Na
+
)
[Ne]
Chl
[
a
b
C06_016A
186 pm
102 pm
181 pm
100 pm
Sodium atom (Na)
[Ne]3s
1
Sodium ion (Na
+
)
[Ne]
Chlorine atom (Cl)
[Ne]3s
2
3p
5
Chlorine ion (Cl )
[Ne]3s
2
3p
6
or [Ar]
a
b
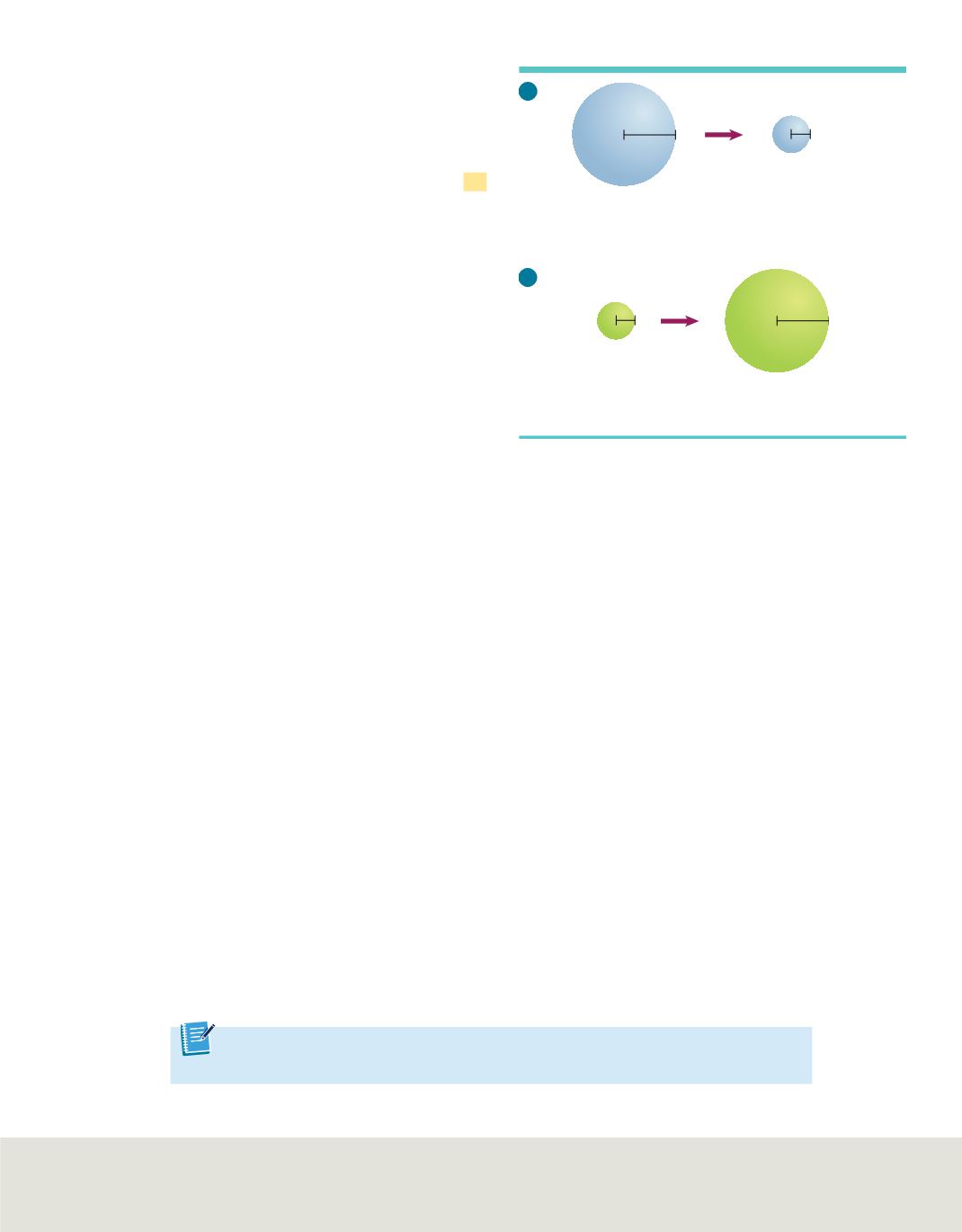
Ionic Radius
Atoms can gain or lose one or more elec-
trons to form ions. Because electrons are
negatively charged, atoms that gain or lose
electrons acquire a net charge. Thus, an
ion
is an atom or a bonded group of atoms that
has a positive or negative charge.
You will learn more about ions later, but for
now, consider how the formation of an ion
affects the size of an atom.
Losing electrons
When atoms lose electrons and form
positively charged ions, they always
become smaller. The reason is twofold. The
electron lost from the atom will almost
always be a valence electron. The loss of a
valence electron can leave a completely
empty outer orbital, which results in a
smaller radius. Furthermore, the electro-
static repulsion between the now-fewer
number of remaining electrons decreases.
As a result, they experience a greater
nuclear charge allowing these remaining electrons to
be pulled closer to the positively charged nucleus.
Figure 14a
illustrates how the radius of sodium decreases when sodium atoms form
positive ions. The outer orbital of the sodium atom is unoccupied in the sodium ion, so
the sodium ion is much smaller than the sodium atom.
Gaining electrons
When atoms gain electrons and form negatively charged ions, they become larger. The
addition of an electron to an atom increases the electrostatic repulsion between the
atom’s outer electrons, forcing them to move farther apart. The increased distance
between the outer electrons results in a larger radius.
Figure 14b
shows how the radius of chlorine increases when chlorine atoms form
negative ions. Adding an electron to a chlorine atom increases the electrostatic repul-
sion among its valence electrons. The increased repulsion causes the electrons to move
farther apart and results in the radius of a chloride ion being almost twice as large as
that of a chlorine atom.
Figure 14
The size of atoms varies greatly when they
form ions.
a.
Positive ions are smaller than the neutral atoms from which
they form.
b.
Negative ions are larger than the neutral atoms from which
they form.
Get It?
Explain
why a lithium ion is smaller than a lithium atom.
Lesson 3 • Periodic Trends
155









