
C06_014A
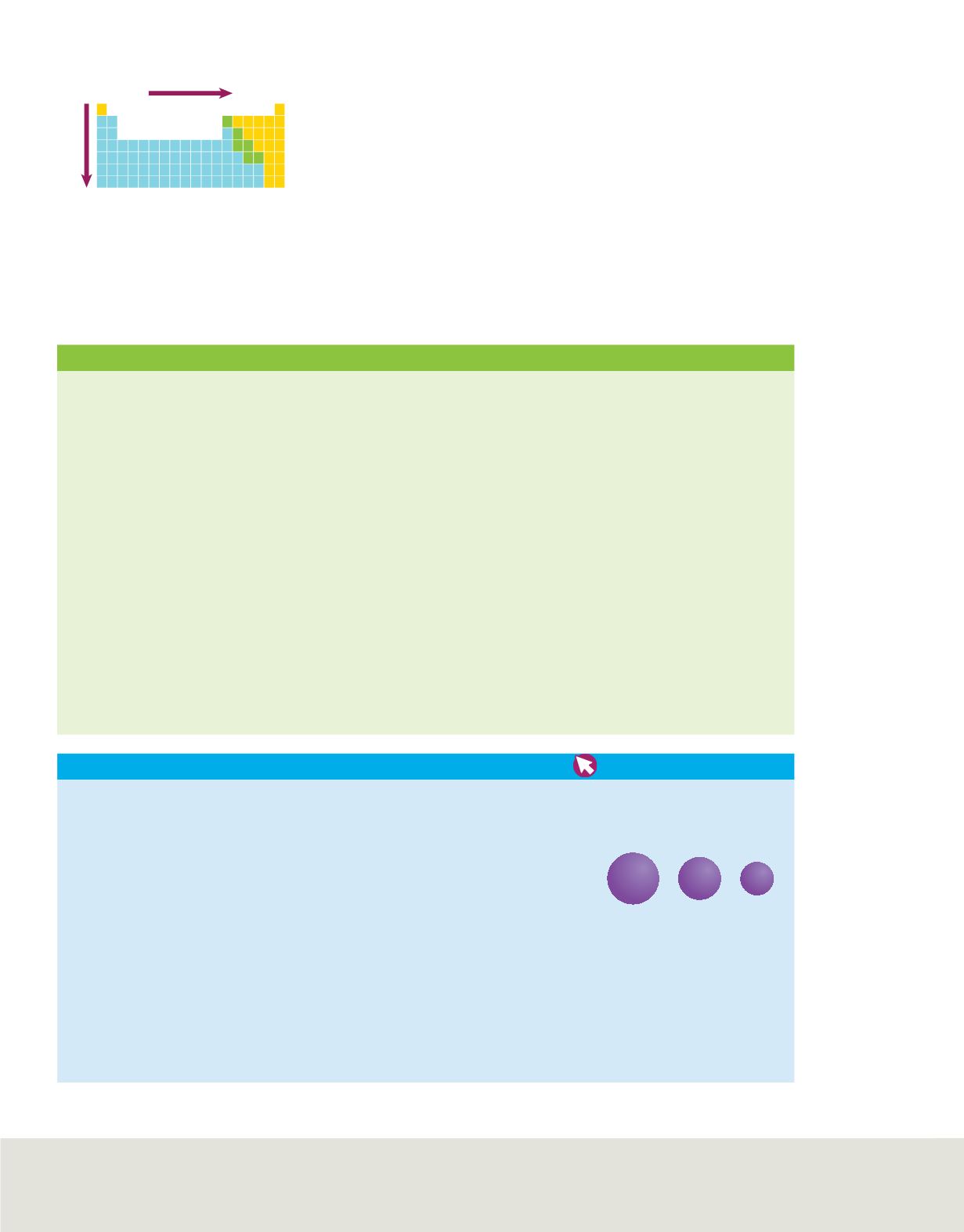
Trends in Atomic Radii
Generally decreases
Generally increases
Figure 13
Atomic radii generally
decrease from left to right in a period
and generally increase as you move
down a group.
EXAMPLE
Problem 2
ELECTRON CONFIGURATION AND THE PERIODIC TABLE
Which has the largest atomic radius:
carbon (C), fluorine (F), beryllium (Be), or lithium (Li)? Answer without referring to
Figure 12
. Explain
your answer in terms of trends in atomic radii.
1
ANALYZE THE PROBLEM
You are given four elements. First, determine the groups and periods the elements occupy.
Then apply the general trends in atomic radii to determine which has the largest atomic radius.
2
SOLVE FOR THE UNKNOWN
From the periodic table, all the elements are found to be in period 2.
Ordering the elements from left-to-right across the period yields:
Li, Be, C, and F.
The first element in period 2, lithium, has the largest radius.
3
EVALUATE THE ANSWER
The period trend in atomic radii has been correctly applied. Checking radii
values in
Figure 12
verifies the answer.
Determine the periods.
Apply the trend of decreasing
radii across a period.
PRACTICE
Problems
Answer the following questions using your knowledge of group and period trends in atomic radii.
Do not use the atomic radii values in Figure 12 to answer the questions.
16.
Which has the largest atomic radius: magnesium (Mg), silicon (Si), sulfur (S),
or sodium (Na)? The smallest?
17.
The figure on the right shows helium, krypton, and radon. Which
one is krypton? How can you tell?
18.
Can you determine which of two unknown elements has the larger
radius if the only known information is that the atomic number of one
of the elements is 20 greater than the other? Explain.
19. CHALLENGE
Determine which element in each pair has the largest atomic radius:
a.
the element in period 2, group 1; or the element in period 3, group 18
b.
the element in period 5, group 2; or the element in period 3, group 16
c.
the element in period 3, group 14; or the element in period 6, group 15
d.
the element in period 4, group 18; or the element in period 2, group 16
A
B C
C06_015A
ADDITIONAL PRACTICE
Moving down a group, the outermost orbital
increases in size along with the increasing
principal energy level; thus, the atom becomes
larger. The larger orbital means that the outer
electrons are farther from the nucleus. This
increased distance offsets the pull of the
increased nuclear charge. Also, as additional
orbitals between the nucleus and the outer
electrons are occupied, these electrons shield the
outer electrons from the nucleus.
Figure 13
summarizes the group and period trends.
154
Module 5 • The Periodic Table and Periodic Law









