
C06_019A
0
10
20
30
40
50
60
First ionization energy (kJ/mol)
2500
2000
1500
1000
500
0
Atomic number
First Ionization Energy of Elements in Periods 1–5
Period 4
Period 5
Period 3
Period 2
Li
K
H
Na
Rb
He
Ne
Ar
Kr
Xe
Get It?
Define
ionization energy
.
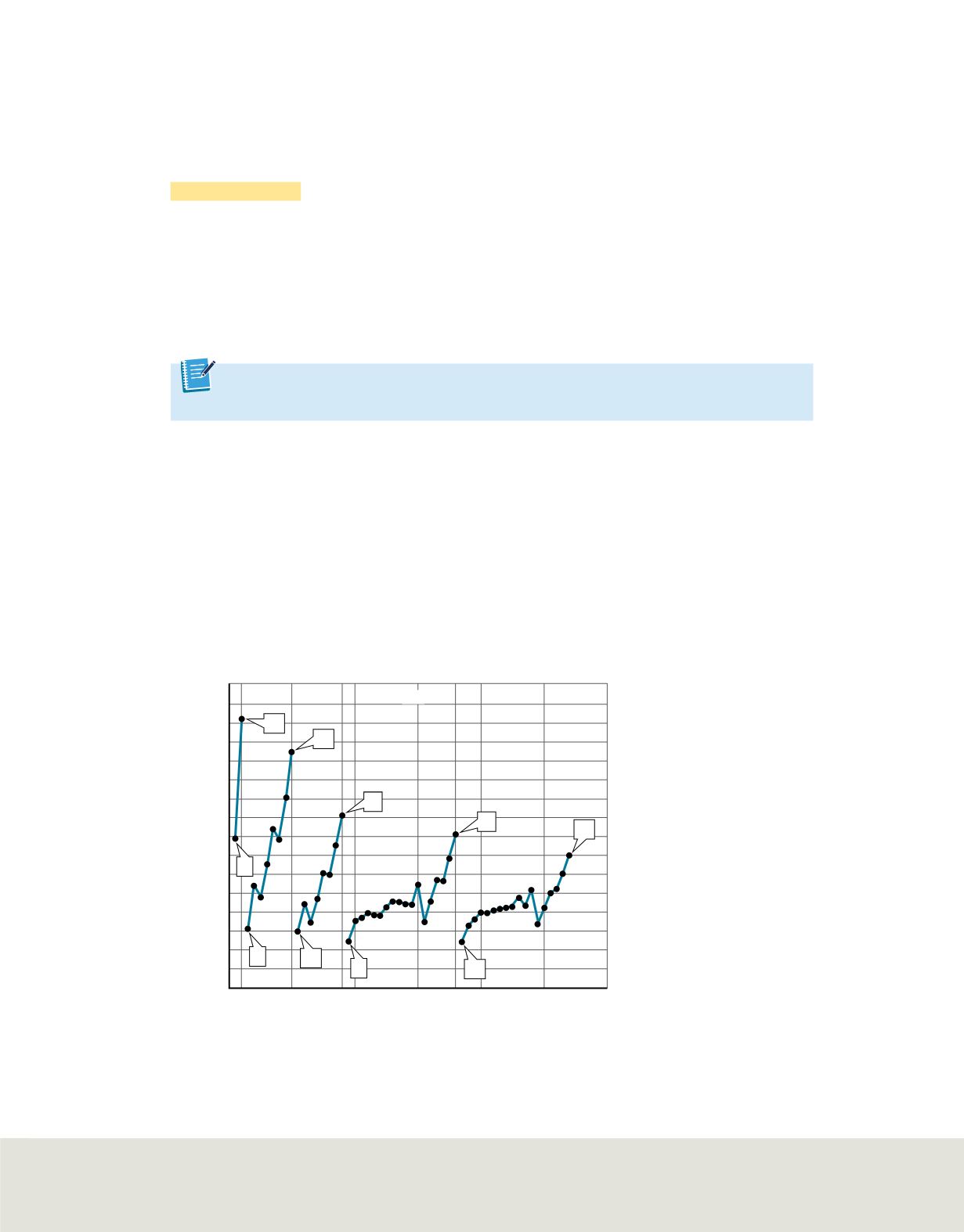
Figure 17
The first ionization energies for elements in periods 1 through 5
are shown as a function of the atomic number.
Describe
how ionization energy and atomic number are related as shown
on this scatter plot.
Ionization Energy
To form a positive ion, an electron must be removed from a neutral atom. This requires
energy. The energy is needed to overcome the attraction between the positive charge of
the nucleus and the negative charge of the electron.
Ionization energy
is defined as the energy required to remove an electron from a
gaseous atom. For example, 8.64
×
10
–
19
J is required to remove an electron from a
gaseous lithium atom. The energy required to remove the first outermost electron from
an atom is called the first ionization energy. The first ionization energy of lithium equals
8.64
×
10
–
19
J. The loss of the electron results in the formation of a Li
+
ion. The first
ionization energies of the elements in periods 1 through 5 are plotted on the graph in
Figure 17
.
Think of ionization energy as an indication of how strongly an atom’s nucleus holds
onto its valence electrons. A high ionization energy value indicates the atom has a
strong hold on its electrons. Atoms with large ionization energy values are less likely to
form positive ions. Likewise, a low ionization energy value indicates an atom loses an
outer electron easily. Such atoms are likely to form positive ions. Lithium’s low ioniza-
tion energy, for example, is important for its use in lithium-ion computer backup
batteries, where the ability to lose electrons easily makes a battery that can quickly
provide a large amount of electrical power.
Lesson 3 • Periodic Trends
157









