
Each set of connected points on the graph in
Figure 17
represents the elements in a
period. The group 1 metals have low ionization energies. Thus, group 1 metals (Li, Na,
K, Rb) are likely to form positive ions. The group 18 elements (He, Ne, Ar, Kr, Xe) have
high ionization energies and are unlikely to form ions. The stable electron configuration
of gases of group 18 greatly limits their reactivity.
Removing more than one electron
After removing the first electron from an atom, it is possible to remove additional
electrons. The amount of energy required to remove a second electron from a 1
+
ion is
called the second ionization energy, the amount of energy required to remove a third
electron from a 2
+
ion is called the third ionization energy, and so on.
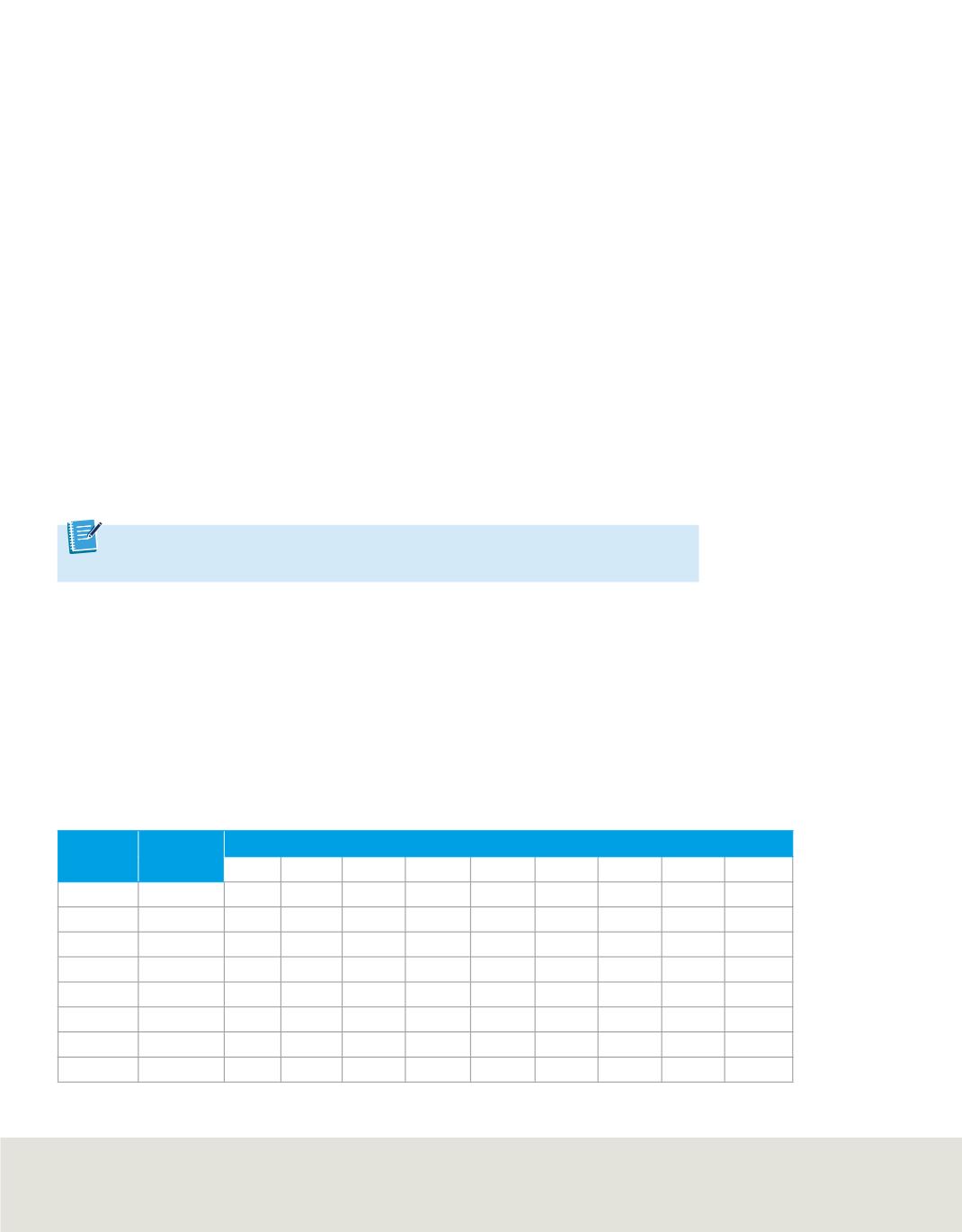
Table 5
lists the
first through ninth ionization energies for elements in period 2.
Reading across
Table 5
from left to right, you will see that the energy required for each
successive ionization always increases. However, the increase in energy does not occur
smoothly. Note that for each element there is an ionization for which the required
energy increases dramatically. For example, the second ionization energy of lithium
(7300 kJ/mol) is much greater than its first ionization energy (520 kJ/mol). This means
that a lithium atom is likely to lose its first valence electron but extremely unlikely to
lose its second.
If you examine the
Table 5
, you will notice that the ionization at which the large
increase in energy occurs is related to the atom’s number of valence electrons. The
element lithium has one valence electron and the increase occurs after the first ioniza-
tion energy. Lithium easily forms the common lithium 1
+
ion but is unlikely to form a
lithium 2
+
ion. The increase in ionization energy shows that atoms hold onto their
inner core electrons much more strongly than they hold onto their valence (outermost)
electrons.
Get It?
Infer
how many electrons carbon is likely to lose.
Table 5
Successive Ionization Energies for the Period 2 Elements
Element Valence
Electrons
Ionization Energy (kJ/mol)*
1
st
2
nd
3
rd
4
th
5
th
6
th
7
th
8
th
9
th
Li
1
520 7300 11,810
Be
2
900 1760 14,850 21,010
B
3
800 2430 3660 25,020 32,820
C
4
1090 2350 4620 6220 37,830 47,280
N
5
1400 2860 4580 7480 9440 53,270 64,360
O
6
1310 3390 5300 7470 10,980 13,330 71,870 84,080
F
7
1680 3370 6050 8410 11,020 15,160 17,870 92,040 106,430
Ne
8
2080 3950 6120 9370 12,180 15,240 20,000 23,070 115,380
*mol is an abbreviation for mole, a quantity of matter
158
Module 5 • The Periodic Table and Periodic Law









