
ulkan/iStock/Getty Images
Figure 5
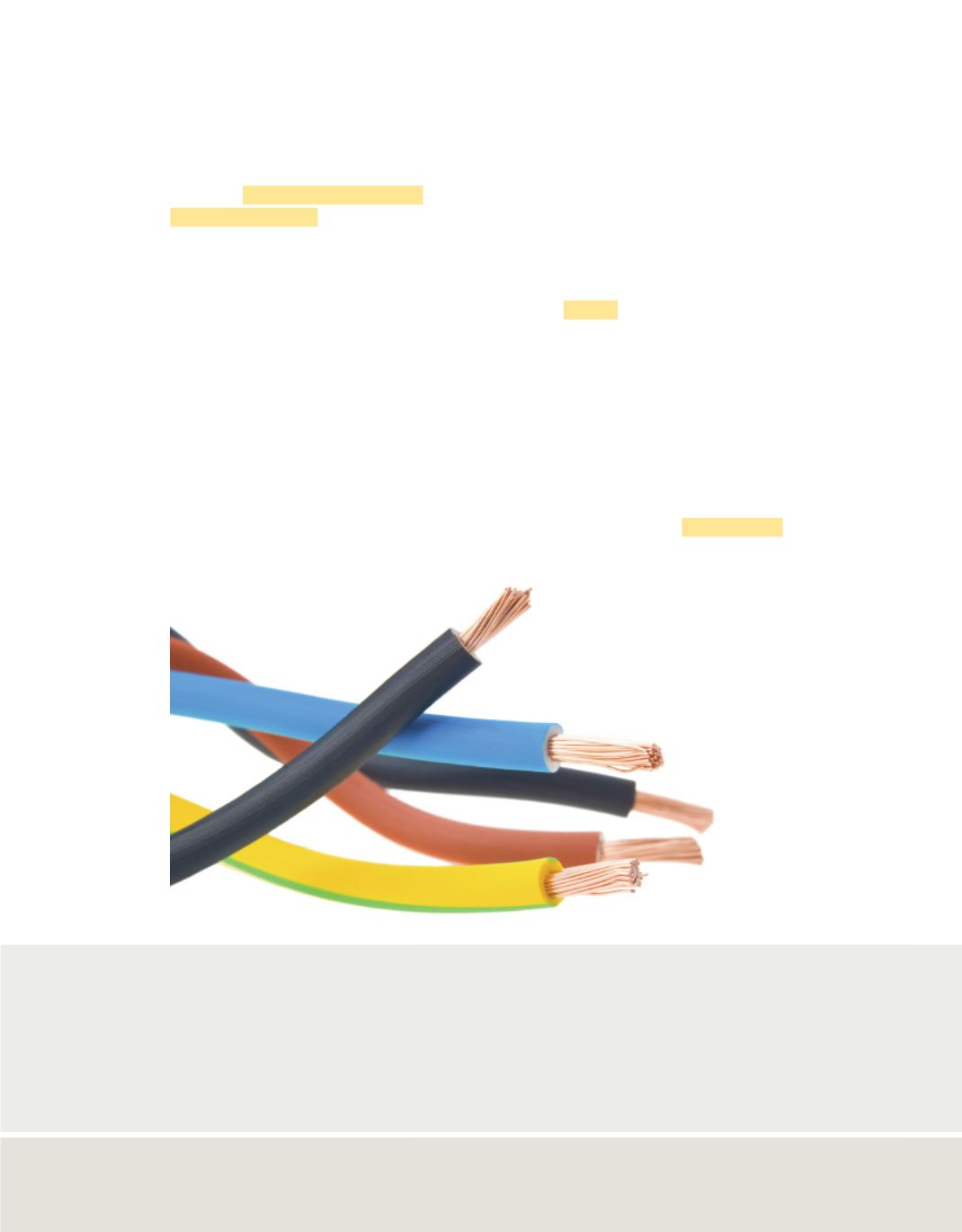
Copper, like most
metals, is ductile and
conducts electricity well.
For these reasons copper is
used for electrical wiring.
Groups and periods
Beginning with hydrogen in period 1, there are a total of seven periods. Each group is
numbered 1 through 18. For example, period 4 contains potassium and calcium. Oxy-
gen is in group 16. The elements in groups 1, 2, and 13 to 18 possess a wide range of
chemical and physical properties. For this reason, they are often referred to as the main
group, or
representative elements.
The elements in groups 3 to 12 are referred to as the
transition elements.
Elements are also classified as metals, nonmetals, and metalloids.
Metals
Elements that are generally shiny when smooth and clean, solid at room temperature,
and good conductors of heat and electricity are called
metals.
Most metals are also
malleable and ductile, meaning that they can be pounded into thin sheets and drawn
into wires, respectively, as shown in
Figure 5
.
Most representative elements and all transition elements are metals. If you look at
boron (B) in column 13, you will see a heavy stairstep line that zigzags down to astatine
(At) at the bottom of group 17. This stairstep line is a visual divider between the metals
and the nonmetals on the table. In the periodic table shown in
Figure 4
metals are
represented by the blue boxes.
Alkali Metals
Except for hydrogen, all of the elements on the left side of the table are
metals. The group 1 elements (except for hydrogen) are known as the
alkali metals.
Because they are so reactive, alkali metals usually exist as compounds with other
elements. Two familiar alkali metals are sodium (Na), one of the components of salt,
and lithium (Li), often used in batteries.
SCIENCE USAGE V. COMMON USAGE
conductor
Science usage:
a substance or body capable of transmitting electricity, heat,
or sound
Copper is a good conductor of heat.
Common usage:
a person who conducts an orchestra, chorus, or other group
of musical performers
The new conductor helped the orchestra perform at its best.
Lesson 1 • Development of the Modern Periodic Table
143









