
C06_003A
H
A 1
F
A
8
Li
B 2
Na
B
9
G
C 3
Mg
C 10
Bo
D 4
Al
D 11
C
E
5
Si
E
12
N
F
6
P
F
13
O
G 7
S
G 14
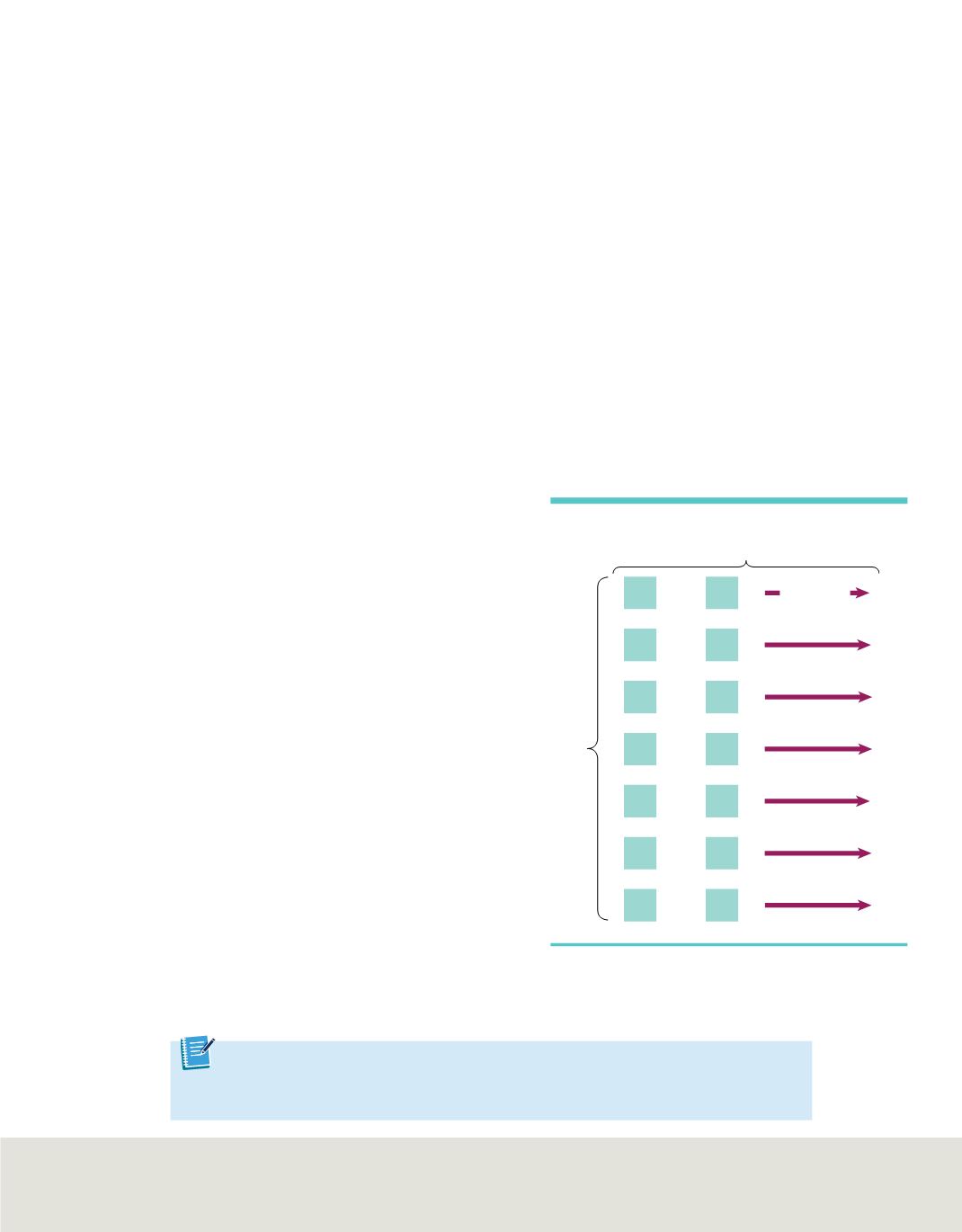
1 octave
Elements with similar properties
are in the same row.
and so on
Figure 1
John Newlands noticed that the properties of
elements repeated every eighth element in the same way
musical notes repeat every eighth note and form octaves.
Get It?
Describe
the pattern that both Newlands and Mendeleev noticed about the
properties of the elements.
Organizing the elements
A significant step toward developing a tool for organizing the elements and the large
amount of data about their properties came in 1860, when chemists agreed upon a
method for accurately determining the atomic masses of the elements. Until this time,
different chemists used different mass values in their work, making the results of one
chemist’s work hard to reproduce by another.
With newly agreed-upon atomic masses for the elements, the search for relationships
between atomic mass and elemental properties, and a way to organize the elements,
began in earnest.
John Newlands
In 1864, English chemist John Newlands (1837–1898) proposed an organizational
scheme for the elements. He noticed that when the elements were arranged by increas-
ing atomic mass, their properties repeated every eighth element. A pattern such as this
is called periodic because it repeats in a specific manner. Newlands named the periodic
relationship that he observed in chemical properties the law of octaves, after the musi-
cal octave in which notes repeat every eighth tone.
Figure 1
shows how Newlands organized 14 of
the elements known in the mid-1860s. Accep-
tance of the law of octaves was hampered because
the law did not work for all of the known ele-
ments. Also, the use of the word octave was
harshly criticized by fellow scientists, who thought
that the musical analogy was unscientific. While
his law was not generally accepted, the passage of
a few years would show that Newlands was
basically correct: the properties of elements do
repeat in a periodic way.
Meyer and Mendeleev
In 1869, German chemist Lothar Meyer
(1830–1895) and Russian chemist Dmitri
Mendeleev (1834–1907) each demonstrated a
connection between atomic mass and the
properties of elements. Mendeleev, however, is
generally given more credit than Meyer because
he published his organizational scheme first.
Like Newlands several years earlier, Mendeleev
noticed that when the elements were ordered by
increasing atomic mass, there was a periodic
pattern in their properties.
Lesson 1 • Development of the Modern Periodic Table
139









