
Cucu Remus/iStock/Getty Images
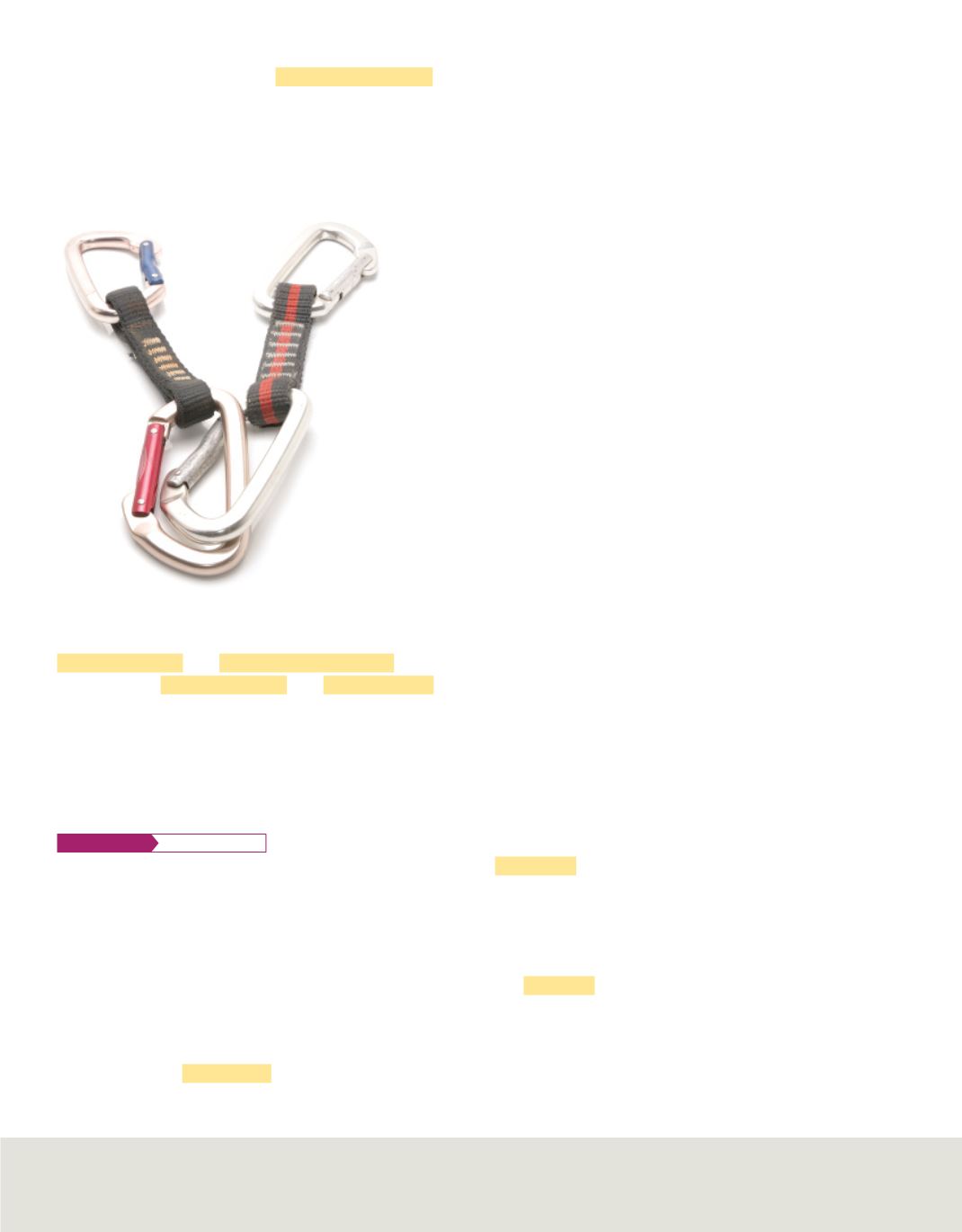
Figure 6
Because magnesium is light and strong,
it is often used in the production of safety devices
such as these caribiners used by climbers.
Alkaline Earth Metals
The
alkaline earth metals
are in group 2. They are also highly
reactive. Calcium (Ca) and magnesium (Mg), two minerals important for your health,
are examples of alkaline earth metals. Because magnesium is strong and relatively
light, it is used in applications in which strength and low mass are important, as shown
in
Figure 6
.
Transition and Inner Transition Metals
The transition elements are divided into
transition metals
and
inner transition metals.
The two sets of inner transition metals,
known as the
lanthanide series
and
actinide series,
are located along the bottom of the
periodic table. The rest of the elements in groups 3 to 12 make up the transition metals.
Elements from the lanthanide series are used extensively as phosphors, substances that
emit light when struck by electrons. Because it is strong and light, the transition metal
titanium is used to make frames for bicycles and eyeglasses.
Nonmetals
BIOLOGY
Connection
Nonmetals occupy the upper-right side of the periodic
table. They are represented by the yellow boxes in
Figure 4
.
Nonmetals
are elements
that are generally gases or brittle, dull-looking solids. They are poor conductors of heat
and electricity. The only nonmetal that is a liquid at room temperature is bromine (Br).
The most abundant element in the human body is the nonmetal oxygen, which consti-
tutes 65% of the body mass.
Group 17 comprises highly reactive elements that are known as
halogens.
Like the
group 1 and group 2 elements, the halogens are often part of compounds. Compounds
made with the halogen fluorine (F) are commonly added to toothpaste and drinking
water to prevent tooth decay. The extremely unreactive group 18 elements are com-
monly called the
noble gases.
They are used in applications where their unreactivity is
an advantage, such as in lasers, a variety of light bulbs, and neon signs.
144
Module 5 • The Periodic Table and Periodic Law









