
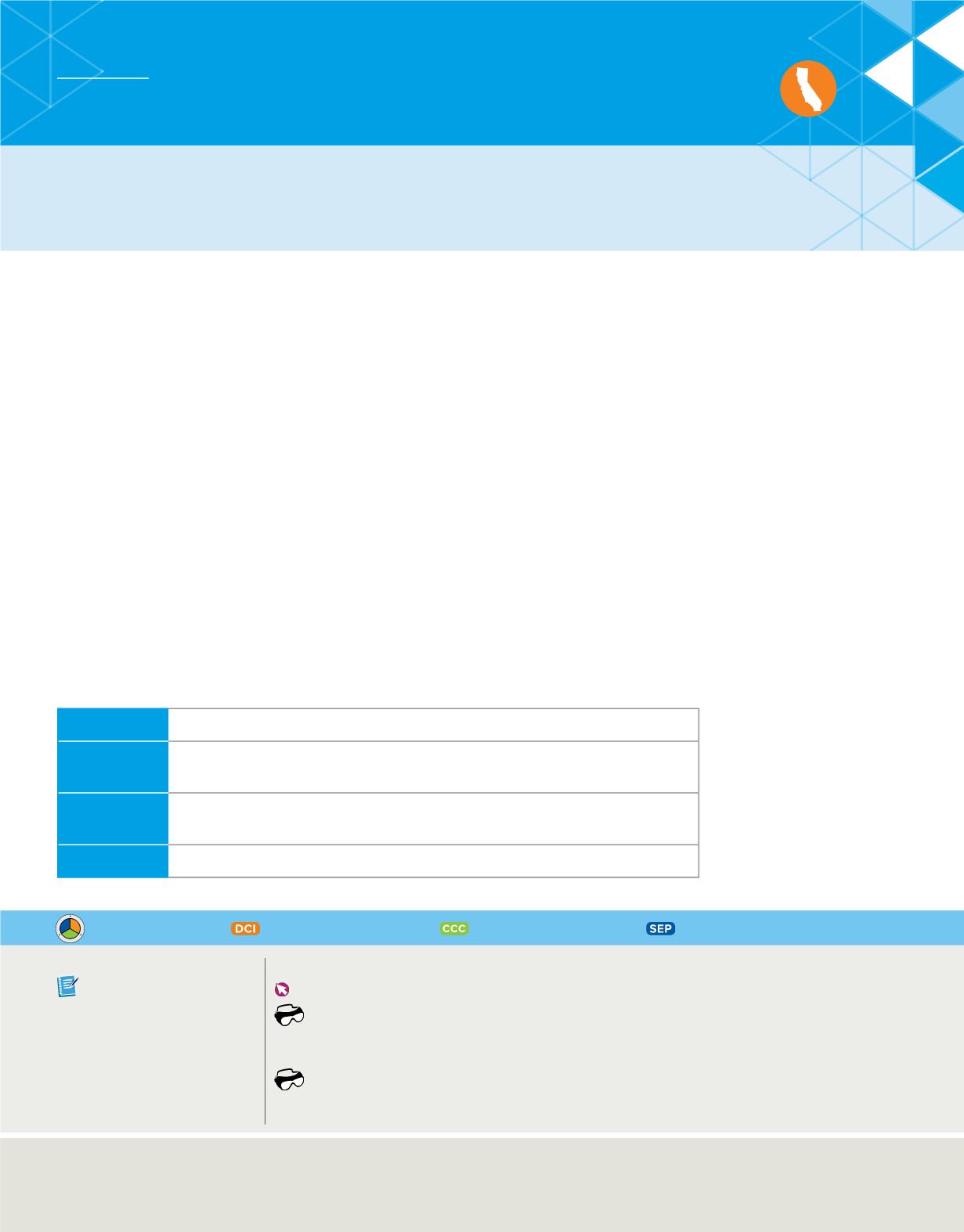
Table 1
Lavoisier’s Table of Simple Substances (Old English Names)
Gases
light, heat, dephlogisticated air, phlogisticated gas, inflammable air
Metals
antimony, silver, arsenic, bismuth, cobalt, copper, tin, iron, manganese,
mercury, molybdena, nickel, gold, platina, lead, tungsten, zinc
Nonmetals
sulphur, phosphorus, pure charcoal, radical muriatique*, radical fluorique*,
radical boracique*
Earths
chalk, magnesia, barote, clay, siliceous earth
*no English name
Development of the Periodic Table
In the late 1700s, French scientist Antoine Lavoisier (1743–1794) compiled a list of all
elements that were known at the time. The list, shown in
Table 1
, contained 33 elements
organized in four categories. Many of these elements, such as silver, gold, carbon, and
oxygen, have been known since prehistoric times.
The 1800s brought a large increase in the number of known elements. The advent of
electricity, which was used to break down compounds into their components, and the
development of the spectrometer, which was used to identify the newly isolated elements,
played major roles in the advancement of chemistry. The industrial revolution of the mid-
1800s also played a major role, which led to the development of many new chemistry-based
industries, such as the manufacture of petrochemicals, soaps, dyes, and fertilizers. By 1870,
there were over 60 known elements.
Along with the discovery of new elements came volumes of new scientific data related
to the elements and their compounds. Chemists of the time were overwhelmed with
learning the properties of so many new elements and compounds. What chemists
needed was a tool for organizing the many facts associated with the elements.
FOCUS QUESTION
How are elements organized in the periodic table?
LESSON 1
DEVELOPMENT OF THE MODERN PERIODIC TABLE
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Virtual Investigation:
Periodic Properties
Analyze and interpret the data
in the periodic table of elements for
patterns
of
organization
and the properties of matter.
ChemLAB:
Investigate Descriptive Chemistry
Analyze and interpret data
to determine
patterns
of properties in representative
elements.
138
Module 5 • The Periodic Table and Periodic Law









