
C06_009A
6
5
4
3
2
2
13 14 15 16 17
1
H
B
Li
Na
Rb
Cs
Be
Mg
Al
Ga
Tl
Si
Cl
Ge
Sn
Pb
As
Sb
Bi
Se
Te
Po
Br
Rn
Xe
Kr
Ar
Ne
He
Ca
Sr
Ba
C N O
P S
F
K
18
1
In
I
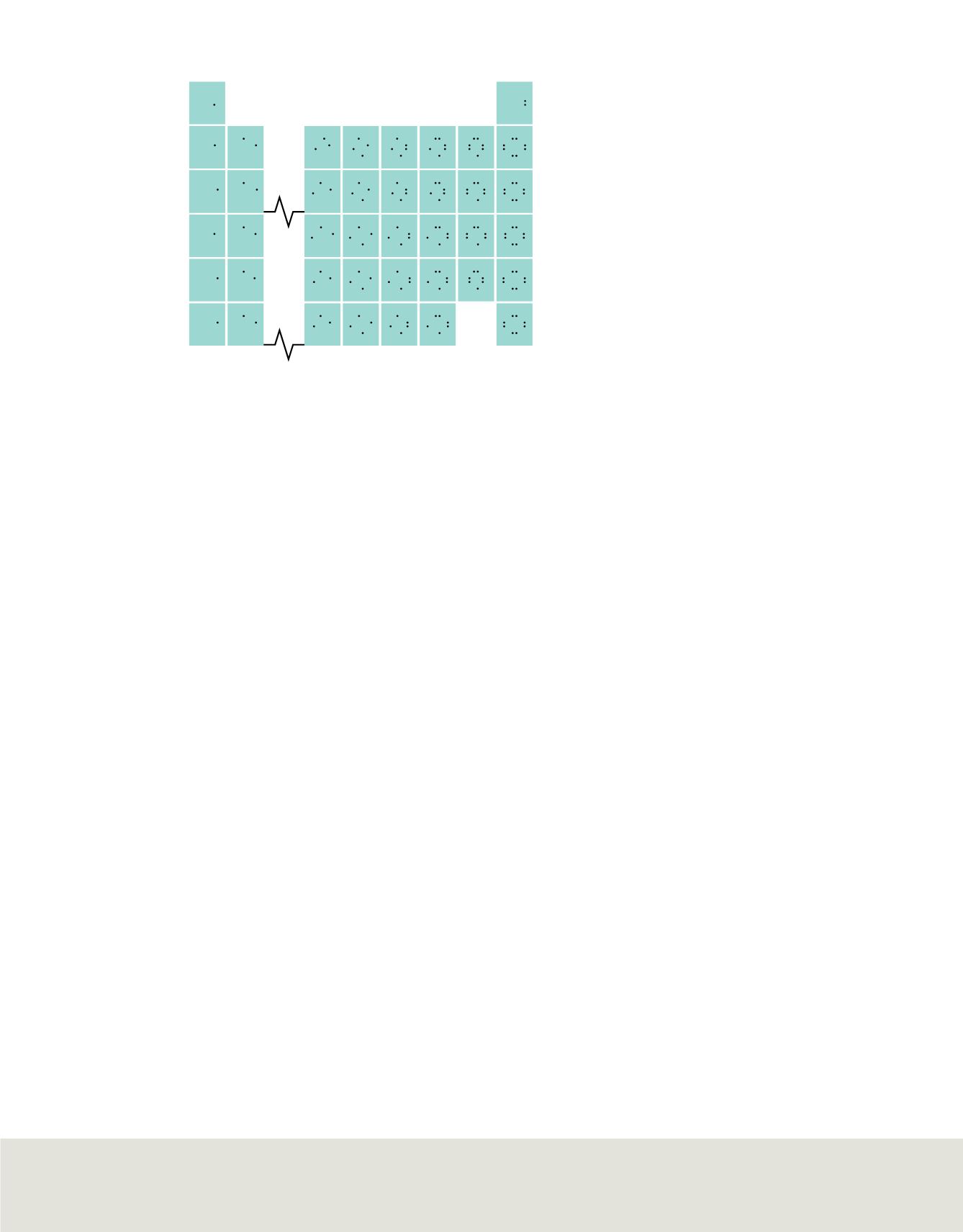
Figure 8
The figure shows the electron-dot
structure of most representative elements.
Observe
How does the number of valence
electrons vary within a group?
Valence electrons and period
The energy level of an element’s valence electrons indicates the period on the periodic
table in which it is found. For example, lithium’s valence electron is in the second
energy level and lithium is found in period 2. Now look at gallium, with its electron
configuration of [Ar]4s
2
3d
10
4p
1
. Gallium’s valence electrons are in the fourth energy
level, and gallium is found in the fourth period.
Valence electrons of the representative elements
Elements in group 1 have one valence electron; group 2 elements have two valence
electrons. Group 13 elements have three valence electrons, group 14 elements have
four, and so on. The noble gases in group 18 each have eight valence electrons, with the
exception of helium, which has only two valence electrons.
Figure 8
shows how the
electron-dot structures you studied previously illustrate the connection between group
number and number of valence electrons. Notice that the number of valence electrons
for the elements in groups 13 to 18 is ten less than their group number.
The s-, p-, d-, and f-Block Elements
The periodic table has columns and rows of varying sizes. The reason behind the table’s
odd shape becomes clear if it is divided into sections, or blocks, representing the atom’s
energy sublevel being filled with valence electrons. Because there are four different
energy sublevels (s, p, d, and f), the periodic table is divided into four distinct blocks, as
shown in
Figure 9
on the next page.
s-Block elements
The s-block consists of groups 1 and 2, and the element helium. Group 1 elements have
partially filled s orbitals containing one valence electron and electron configurations
ending in s
1
. Group 2 elements have completely filled s orbitals containing two valence
electrons and electron configurations ending in s
2
. Because s orbitals hold two electrons
at most, the s-block spans two groups.
Lesson 2 • Classification of the Elements
147









