
190 192
196
200
202 204
194
198
206 208 210
5
0
10
15
20
25
30
Percent abundance
Atomic mass (amu)
Monty Rakusen/Cultura/Getty Images
REVISIT THE PHENOMENON
What is matter made of?
CER
Claim, Evidence, Reasoning
Explain Your Reasoning
Revisit the claim you made when you encountered the
phenomenon. Summarize the evidence you gathered from your investigations and
research and finalize your Summary Table. Does your evidence support your claim? If not,
revise your claim. Explain why your evidence supports your claim.
STEM UNIT PROJECT
Now that you’ve completed the module, revisit your STEM unit project. You will
summarize your evidence and apply it to the project.
Module Wrap-Up
C
C
C
S
E
P
D
C
I
THREE-DIMENSIONAL THINKING
GO FURTHER
Data Analysis Lab
How can you identify an element from mass spectrometer
data?
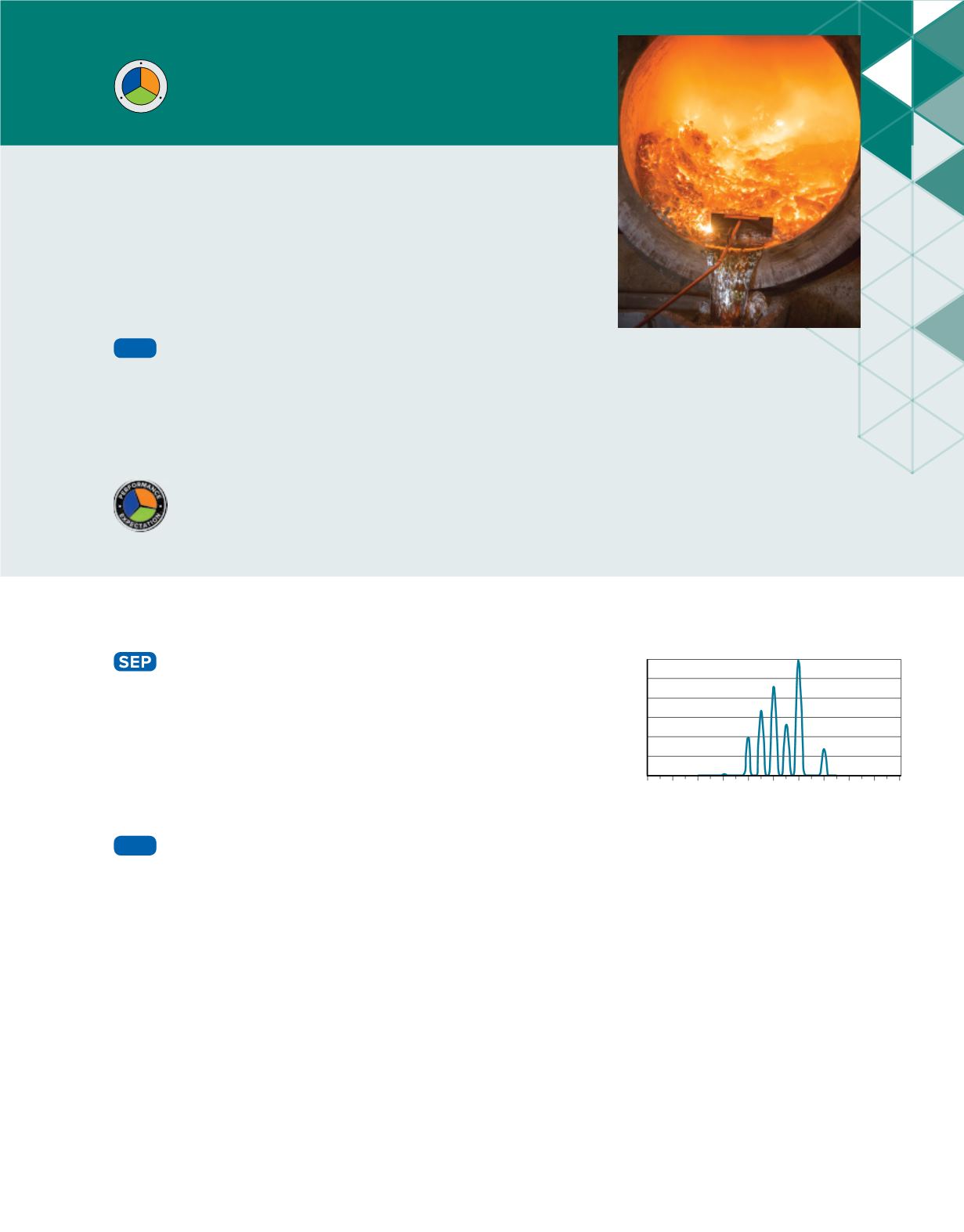
A mass spectrometer separates atoms and molecules
according to their masses. A substance is heated in a
vacuum and then ionized. The ions are accelerated through
a magnetic field, which separates ions of different masses.
CER
Analyze and Interpret Data
1.
Claim, Evidence, Reasoning
Without performing any
calculations, predict the approximate atomic mass of
element X. Explain the reasoning behind your prediction.
2.
Evidence, Reasoning
Calculate the weighted average
mass of the isotopes of element X. Then, use the
periodic table to identify the element.
Module 3 • The Structure of the Atom
103









