
C04_028A
17e
CI
35
17
17e
CI
37
17
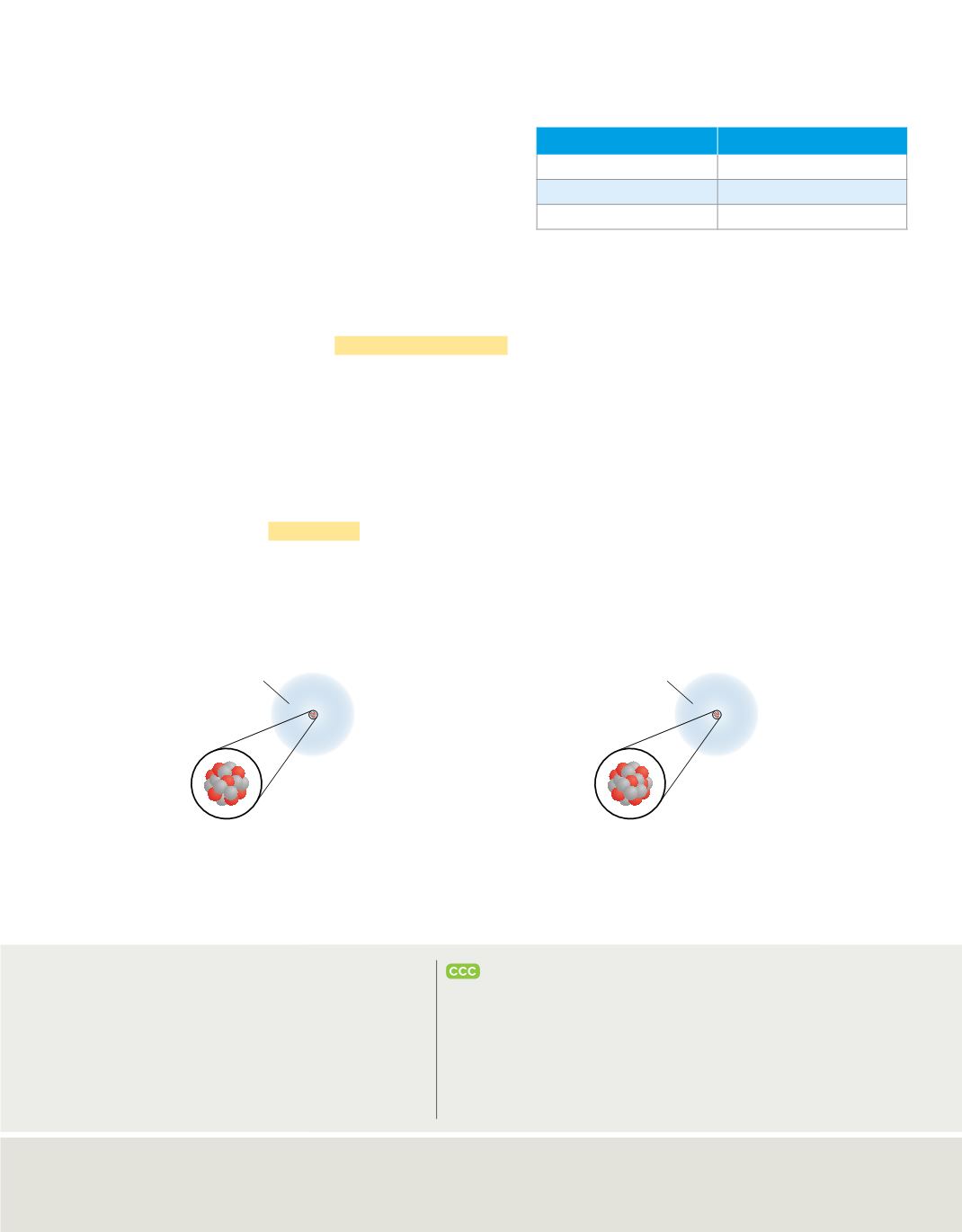
Calculate the Weighted Average Atomic Mass of Chlorine
17p
18n
Atomic mass: 34.969 amu
Percent abundance: 75.78%
Mass contribution:
(34.969 amu)(75.78%)
=
26.50 amu
Weighted average atomic mass of chlorine
=
(26.50 amu
+
8.953 amu)
=
35.45 amu
17p
20n
Atomic mass: 36.966 amu
Percent abundance: 24.22%
Mass contribution:
(36.966 amu)(24.22%)
=
8.953 amu
Table 4
Masses of Subatomic Particles
Particle
Mass (amu)
Electron
0.000549
Proton
1.007276
Neutron
1.008665
Mass of Atoms
Recall from
Table 3
that the masses of both
protons and neutrons are approximately
1.67
×
10
−24
g. While this is a small mass, the
mass of an electron is even smaller—only about
1/1840 that of a proton or a neutron.
Atomic mass unit
Because these extremely
small masses expressed in scientific notation
are difficult to work with, chemists have developed a method of measuring the mass of
an atom relative to the mass of a specific atomic standard. That standard is the
carbon-12 atom. Scientists assigned the carbon-12 atom a mass of exactly 12 atomic
mass units. Thus, one
atomic mass unit (amu)
is defined as one-twelfth the mass of a
carbon-12 atom. Although a mass of 1 amu is nearly equal to the mass of a single
proton or a single neutron, it is important to realize that the values are slightly
different.
Table 4
gives the masses of the subatomic particles in terms of amu.
Atomic mass
Because an atom’s mass depends mainly on the number of protons and
neutrons it contains, and because protons and neutrons have masses close to 1 amu,
you might expect the atomic mass of an element to always be nearly a whole number.
However, this is often not the case. The explanation involves how atomic mass is
defined. The
atomic mass
of an element is the weighted average mass of the isotopes of
that element. Because isotopes have different masses, the weighted average is not a
whole number. The calculation of the atomic mass of chlorine is illustrated in
Figure 18
.
Figure 18
To calculate the weighted average atomic mass of chlorine, you first need to calculate the mass
contribution of each isotope.
ACADEMIC VOCABULARY
specific
characterized by a precise formulation or
accurate restriction; Some diseases have
specific symptoms.
CROSSCUTTING CONCEPTS
Patterns
Identify the pattern found in the periodic table as you move
horizontally across the table from potassium to iron by plotting the atomic
number (
x
-axis) vs. the atomic mass (
y
-axis). Describe the trend in your graph,
including an explanation of how the pattern of electrons, neutrons, and
protons accounts for this trend.
Lesson 3 • How Atoms Differ
95









