
Erick Tseng (visiglow.com)/Moment/Getty Images
Table 1
Physical Properties of Common Substances
Substance
Color
State at 25°C Melting Point
(°C)
Boiling Point
(°C)
Density (g/cm
3
)
at 25°C
Oxygen
colorless
gas
–219
–183
0.0013
Mercury
silver
liquid
–39
357
13.5
Water
colorless
liquid
0
100
0.997
Sucrose
white
solid
186
decomposes
1.58
Sodium
chloride
white
solid
801
1465
2.17
Extensive and intensive properties
Table 1
lists several common substances and
their physical properties. Physical properties can be further described as being one of
two types.
Extensive properties
are dependent on the amount of substance present. For
example, mass is an extensive property. Length and volume are also extensive
properties. Density, on the other hand, is an example of an intensive property of matter.
Intensive properties
are independent of the amount of substance present. For example,
the density of a substance (at constant temperature and pressure) is the same no matter
how much substance is present.
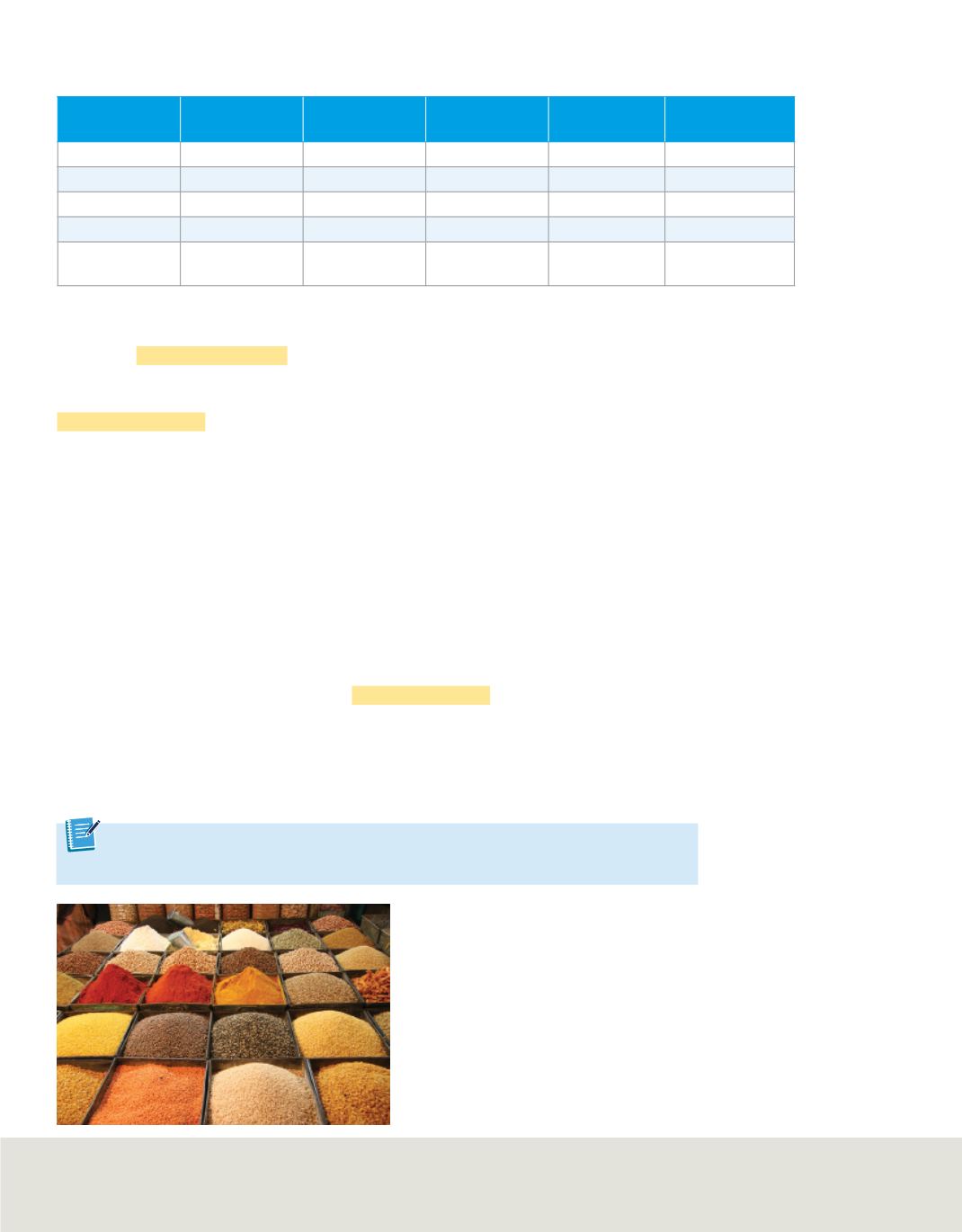
A substance can often be identified by its intensive properties. In some cases, a single
intensive property is unique enough for identification. For instance, most of the spices
shown in
Figure 6
can be identified by their scent.
Chemical Properties of Matter
Some properties of a substance are not obvious unless the substance has changed
composition as a result of its contact with other substances or the application of thermal
or electric energy. The ability or inability of a substance to combine with or change into
one or more other substances is called a
chemical property.
Iron forming rust when combined with the oxygen in air is an example of a chemical
property of iron. Similarly, the inability of a substance to change into another substance
is also a chemical property. For example, when iron is placed in nitrogen gas at room
temperature, no chemical change occurs.
Get It?
Compare
physical and chemical properties.
Figure 6
Many spices can be identified by their
scent, which is an intensive property.
Infer
Name an extensive property of one of the
spices pictured in this figure.
52
Module 2 • Matter—Properties and Changes









