
C03_010A
Gas
C03_008A
Liquid
(t)Andrei Kuzmik/Shutterstock.com, (b)Nelstudio/Shutterstock.com
Figure 4
Gases take the shape and volume of their containers. Particles in a gas are very far apart.
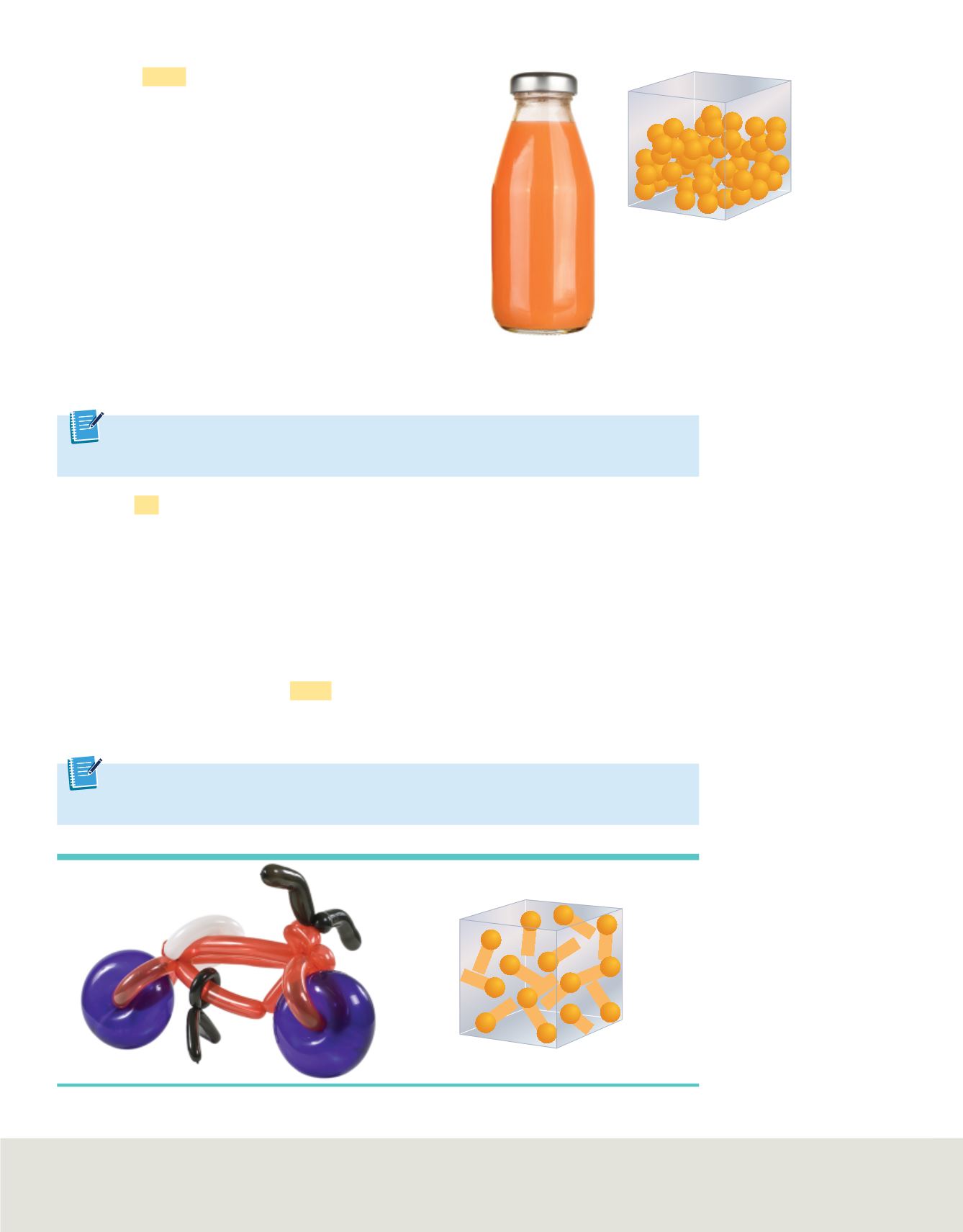
Liquids
A
liquid
is a form of matter that flows, has
constant volume, and takes the shape of its container.
Common examples of liquids include water, blood, and
mercury. The particles in a liquid are not rigidly held in
place and are less closely packed than the particles in a
solid. Liquid particles are able to move past each other.
This property allows a liquid to flow and take the
shape of its container, as shown in
Figure 3
, although it
might not completely fill the container. A liquid’s
volume is constant: regardless of the size and shape of
the container in which the liquid is held, the volume of
the liquid remains the same. Because of the way the
particles of a liquid are packed, liquids are virtually
incompressible. Like solids, however, liquids tend to
expand when they are heated.
Gases
A
gas
is a form of matter that not only flows to conform to the shape of its
container but also fills the entire volume of its container, as shown in
Figure 4
. If you
flow gas into a container and close the container, the gas will expand to fill the
container. Compared to solids and liquids, the particles of gases are far apart. Because
of the significant amount of space between particles, gases are easily compressed.
You are probably familiar with the word
vapor
as it relates to the word
gas
. However, the
words
gas
and
vapor
, while similar, do not mean the same thing, and should not be used
interchangeably. The word
gas
refers to a substance that is naturally in the gaseous state
at room temperature. The word
vapor
refers to the gaseous state of a substance that is a
solid or a liquid at room temperature. For example, steam is a vapor because water
exists as a liquid at room temperature.
Figure 3
A liquid takes the
shape of its container.
Particles in a liquid are not
held in place rigidly.
Get It?
Differentiate
between gas and vapor.
Get It?
Compare
the properties of solids and liquids in terms of their particle arrangements.
50
Module 2 • Matter—Properties and Changes









