
PRACTICE
Problems
5.
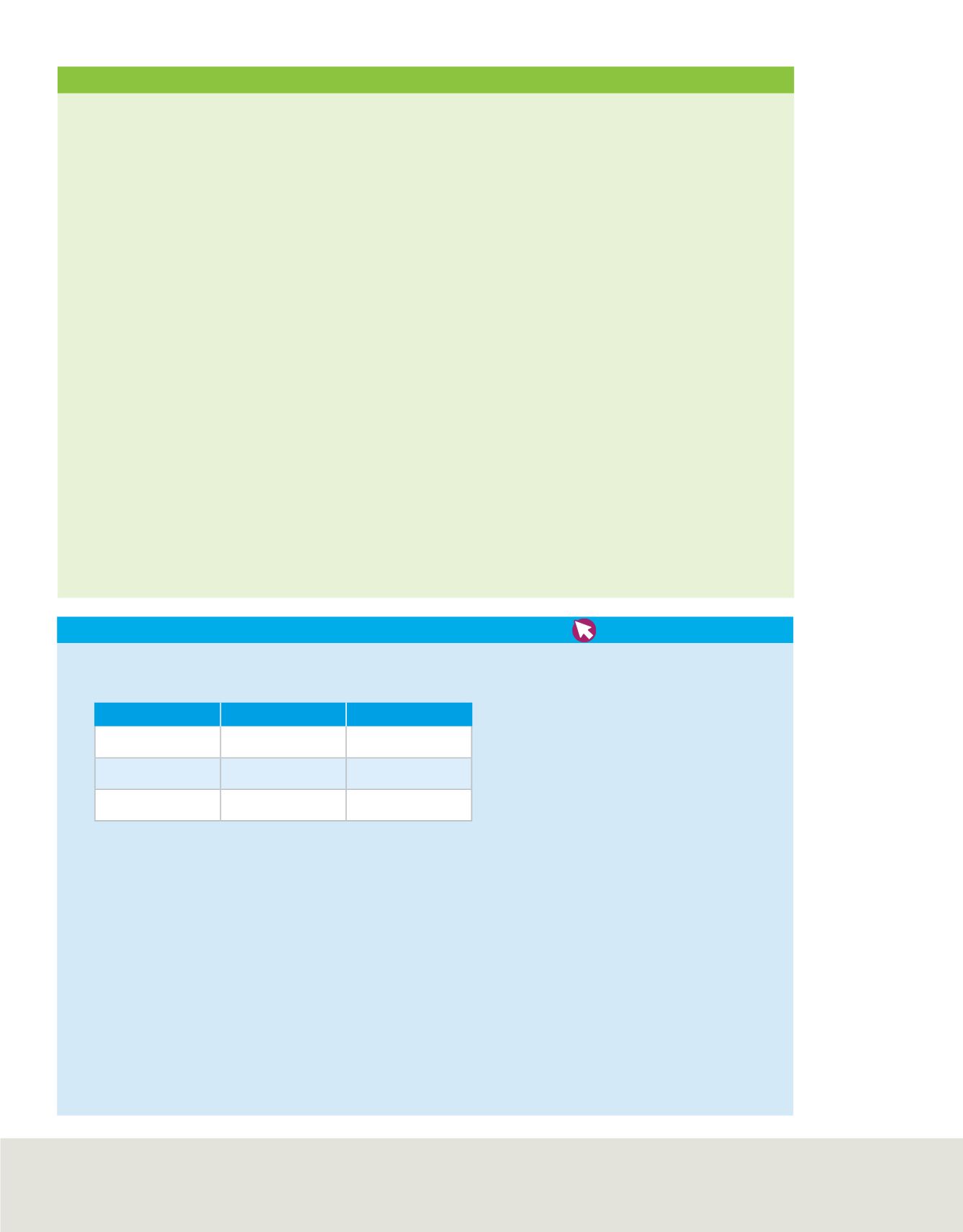
Use the data in the table to answer the following questions.
Aluminum and Liquid Bromine Reaction
Substance
Before Reaction After Reaction
Aluminum
10.3 g
0.0 g
Liquid bromine
100.0 g
8.2 g
Compound
0.0 g
How many grams of bromine reacted? How many grams of compound were formed?
6.
From a laboratory process designed to separate water into hydrogen and oxygen gas, a
student collected 10.0 g of hydrogen and 79.4 g of oxygen. How much water was originally
involved in the process?
7.
A student carefully placed 15.6 g of sodium in a reactor supplied with an excess quantity of
chlorine gas. When the reaction was complete, the student obtained 39.7 g of sodium chloride.
Calculate how many grams of chlorine gas reacted. How many grams of sodium reacted?
8.
A 10.0-g sample of magnesium reacts with oxygen to form 16.6 g of magnesium oxide. How
many grams of oxygen reacted?
9.
CHALLENGE
106.5 g of HCI(g) react with an unknown amount of NH
3
(g) to produce 156.3 g of
NH
4
Cl(s). How many grams of NH
3
(g) reacted? Is the law of conservation of mass observed in
the reaction? Justify your answer.
ADDITIONAL PRACTICE
EXAMPLE
Problem 1
CONSERVATION OF MASS
In an experiment, 10.00 g of red mercury(II) oxide powder is placed in an
open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has
a mass of 9.26 g. What is the mass of oxygen formed in the reaction?
1
ANALYZE THE PROBLEM
You are given the mass of a reactant and the mass of one of the products in a chemical
reaction. According to the law of conservation of mass, the total mass of the products must equal
the total mass of the reactants.
Known
Unknown
m
mercury(II) oxide
=
10.00 g
m
oxygen
=
?
g
m
mercury
=
9.26 g
2
SOLVE FOR THE UNKNOWN
Mass
reactants
=
Mass
products
State the law of conservation of mass.
m
mercury(II) oxide
=
m
mercury
+
m
oxygen
m
oxygen
=
m
mercury(II) oxide
-
m
mercury
Solve for
m
oxygen
m
oxygen
=
10.00 g
-
9.26 g
Substitute
m
mercury(II) oxide
=
10.00 g and
m
mercury
=
9.26 g.
m
oxygen
=
0.74 g
3
EVALUATE THE ANSWER
The sum of the masses of the two products equals the mass of the reactant, verifying that mass
has been conserved. The answer is correctly expressed to the hundredths place, making the
number of significant digits correct.
58
Module 2 • Matter—Properties and Changes









