
(t)Glow Images, (b)Albert Russ/Shutterstock.com
Plasmas
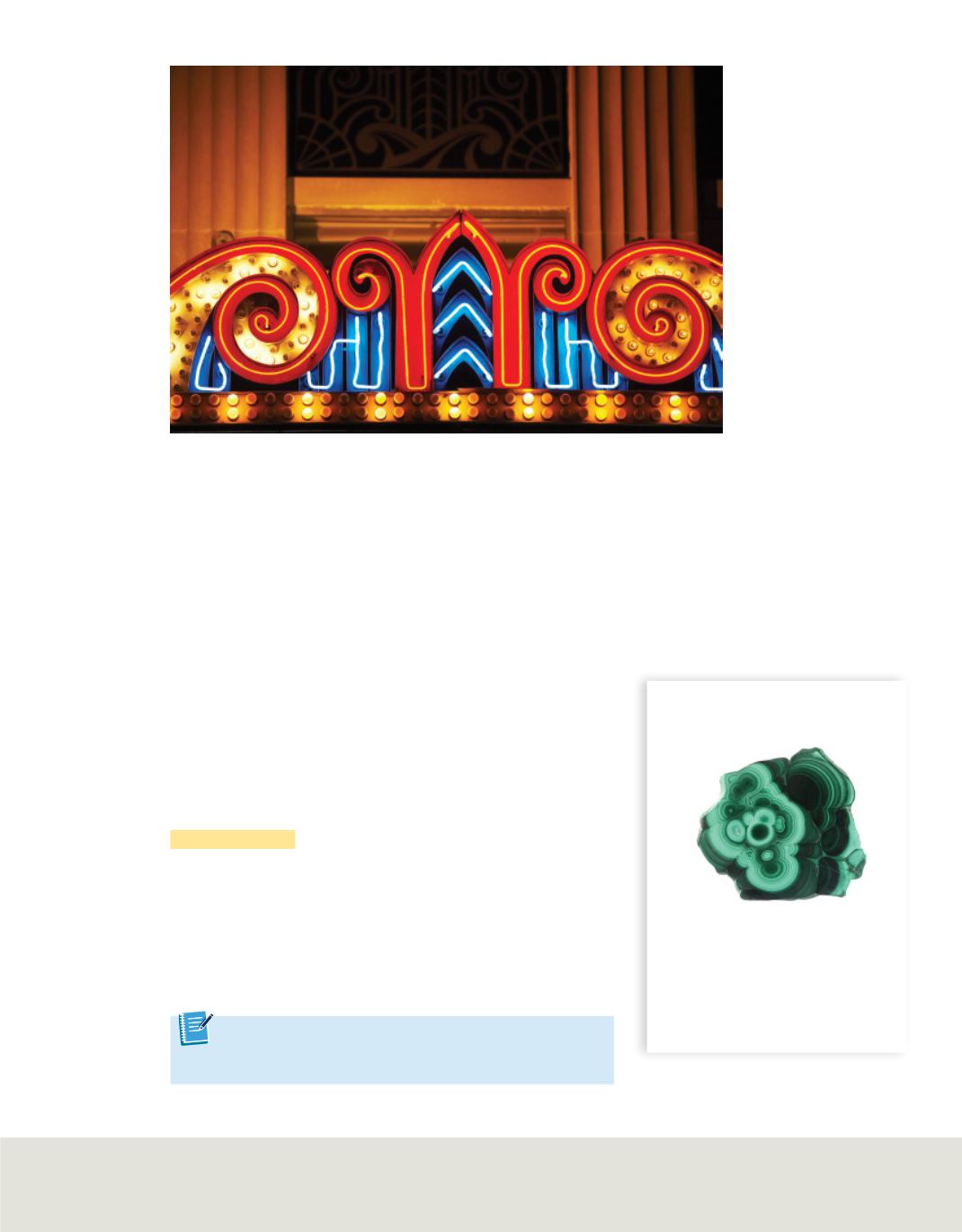
The fluorescent lights in a classroom or the bright, colorful lights in the sign
in
Figure 5
involve matter as a plasma. A plasma is a form of matter that results when
the particles of a gas become ionized and are broken apart into smaller charged
particles. Ionization requires energy, such as that provided by an electric current in a
neon or fluorescent bulb or the high temperatures of lightning or a star. Although
plasmas can behave like gases in many ways, ionization causes plasmas to exhibit a
strong response to electric and magnetic fields.
Real-World Chemistry
Physical Properties
MINERALS Scientists use physical
properties such as color and hardness to
identify minerals. For instance, malachite
is always green and relatively soft.
Malachite was used as a pigment in paint
and is now mainly used to make jewelry.
Physical Properties of Matter
You are probably used to identifying objects by their proper-
ties—their characteristics and behavior. For example, you can
easily identify a pencil in your backpack because you recog-
nize its shape, color, weight, or some other property. These
characteristics are all physical properties of the pencil. A
physical property
is a characteristic of matter that can be
observed or measured without changing the sample’s compo-
sition. Physical properties also describe pure substances.
Because substances have uniform and unchanging composi-
tions, they also have consistent and unchanging physical
properties. Density, color, odor, hardness, melting point, and
boiling point are common physical properties that scientists
record as identifying characteristics of a substance.
Get It?
Define
physical property
and provide examples.
Figure 5
Plasmas are ionized gases.
Lesson 1 • Properties of Matter
51









