
(t)McGraw-Hill Education, (b) Szasz-Fabian Jozsef/Shutterstock.com
Mixtures
You have already read that a pure substance has a uniform and
unchanging composition. What happens when two or more
substances are combined? A
mixture
is a combination of two or
more pure substances in which each pure substance retains its
individual chemical properties. The composition of mixtures is
variable, and the number of mixtures that can be created by
combining substances is infinite. Although much of the focus of
chemistry is the behavior of substances, it is important to
remember that most everyday matter occurs as mixtures.
Substances tend to mix naturally; it is difficult to keep any
substance pure.
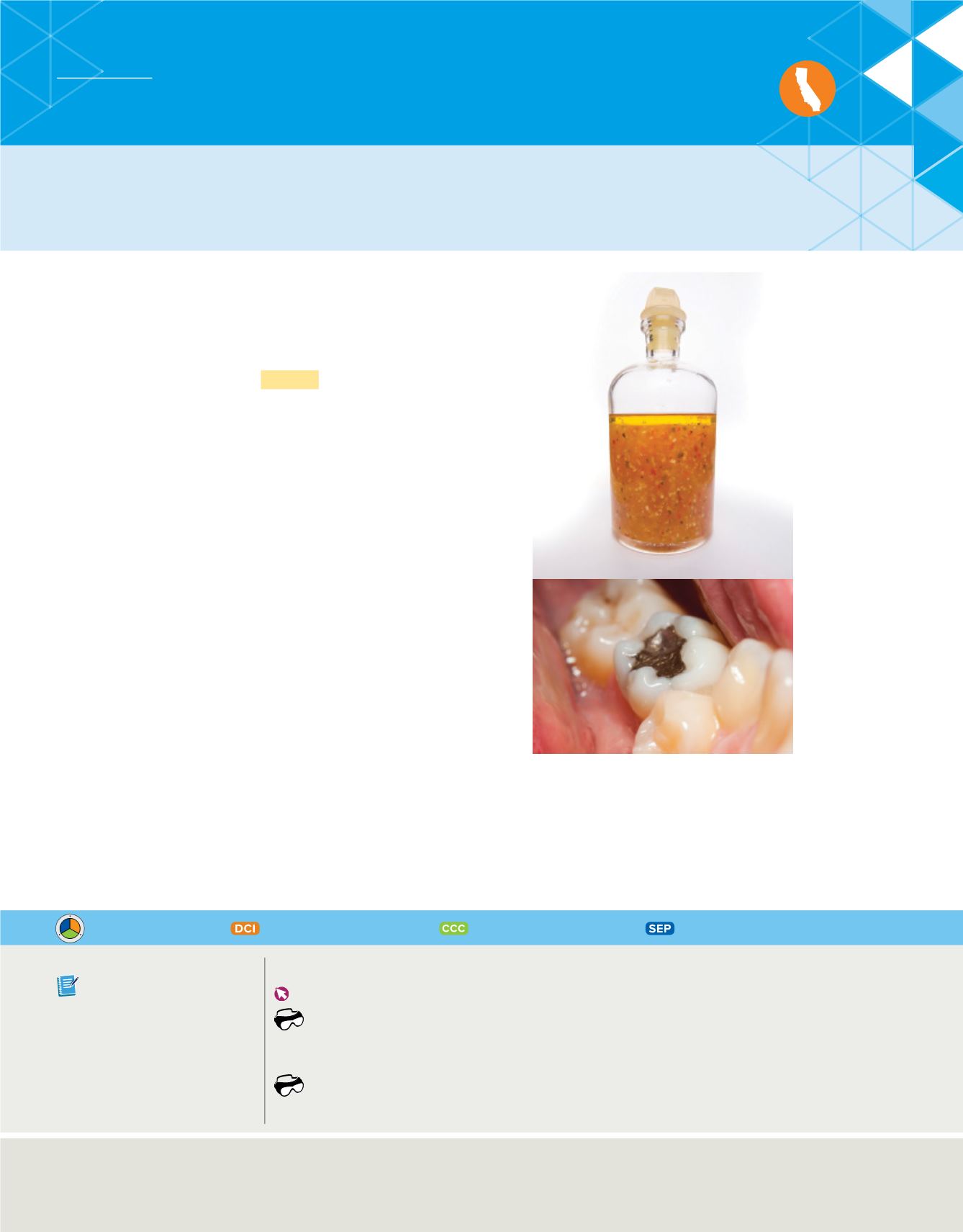
Examine the mixtures in
Figure 19
. When oil, seasonings, and
vinegar are mixed, you can still distinguish all of the compo-
nents. If the mixture remains undisturbed long enough, the oil
will form a layer on top of the vinegar. On the other hand, you
cannot distinguish between the components of the mercury-silver mixture. You could, however, separate them by heating
the mixture. The mercury will evaporate before the silver does,
and you will obtain two separate substances: mercury vapor and
solid silver. The mercury and silver physically mixed to form the
mixture but did not chemically react with each other. They
could be separated by the physical method of boiling. The vapor
can be collected and condensed into liquid mercury.
Figure 19
There are different types of
mixtures. The components of some
mixtures, like this salad dressing, are
visible. It is not possible to see the different
components of some mixtures, such as this
mercury-silver filling.
FOCUS QUESTION
Would the substance still burn blue if it were mixed with
another substance?
LESSON 4
MIXTURES OF MATTER
Disciplinary Core Ideas
Crosscutting Concepts
Science & Engineering Practices
3D THINKING
C
C
C
S
E
P
D
C
I
COLLECT EVIDENCE
Use your Science Journal to
record the evidence you collect as
you complete the readings and
activities in this lesson.
INVESTIGATE
GO ONLINE
to find these activities and more resources.
Small-Scale Lab:
Separation of Aspirin
Carry out an investigation
to determine the
scale, proportion, and quantity
of the
components of a common medication.
Identify Crosscutting Concepts
Create a table of the
crosscutting concepts
and fill in examples you find as you read.
68
Module 2 • Matter—Properties and Changes









