
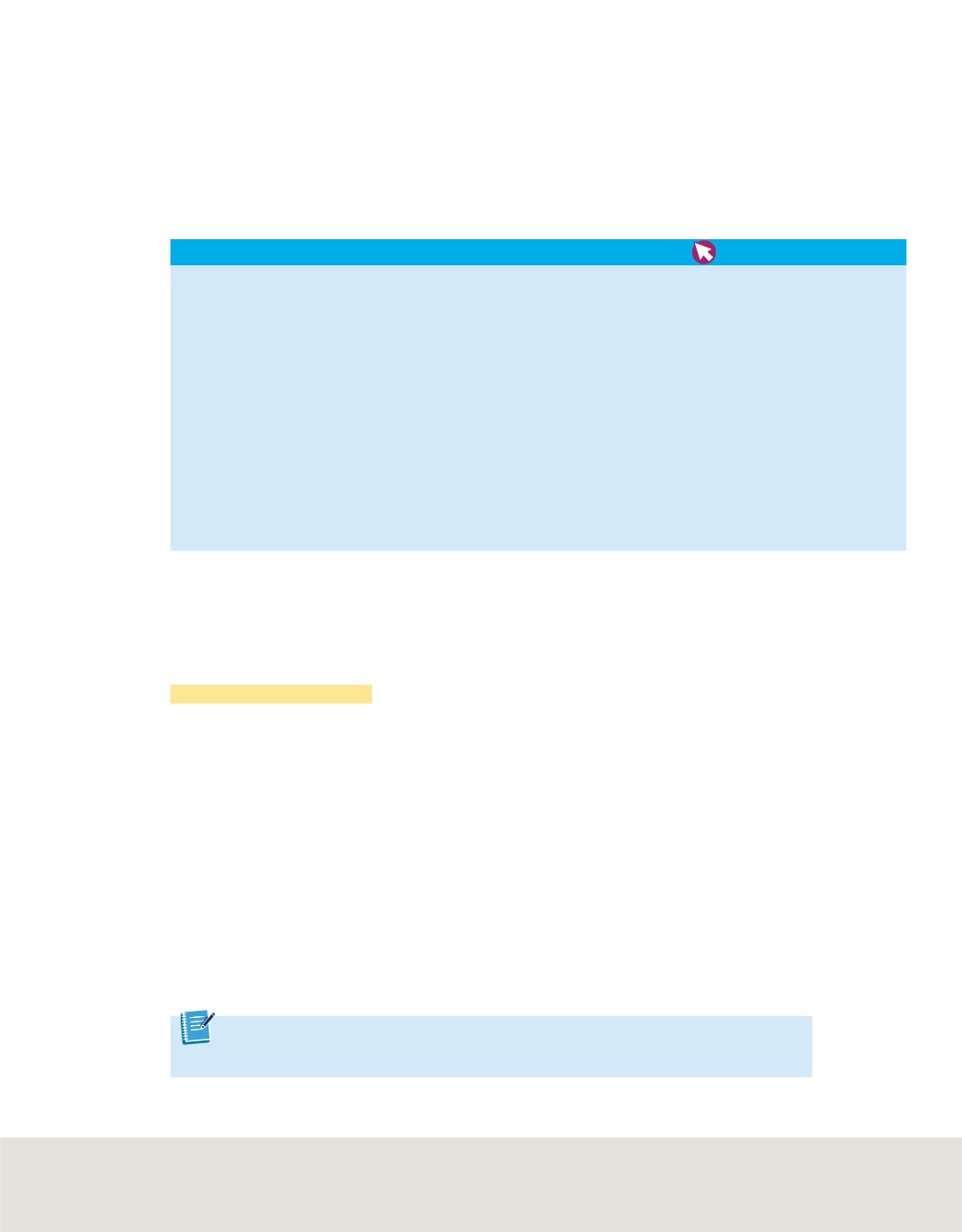
Suppose you analyzed 500.0 g of sucrose from a sample of sugarcane. The analysis is
shown in
Table 3
. The percent-by-mass values for the sugarcane are equal to the values
obtained for the granulated sugar. According to the law of definite proportions, samples
of a compound from any source must have the same mass proportions. Conversely,
compounds with different mass proportions must be different compounds. Thus, you
can conclude that samples of sucrose will always be composed of 42.20% carbon, 6.50%
hydrogen, and 51.30% oxygen, no matter their sources.
Law of Multiple Proportions
Compounds composed of different elements are obviously different compounds.
However, different compounds can also be composed of the same elements. This
happens when those different compounds have different mass compositions. The
law of multiple proportions
states that when different compounds are formed by a
combination of the same elements, different masses of one element combine with
the same fixed mass of the other element in a ratio of small whole numbers. Ratios
compare the relative amounts of any items or substances. The comparison can be
expressed using numbers separated by a colon or as a fraction. With regard to the
law of multiple proportions, ratios express the relationship of elements in a
compound.
Water and hydrogen peroxide
The two distinct compounds water (H
2
O) and
hydrogen peroxide (H
2
O
2
) illustrate the law of multiple proportions. Each compound
contains the same elements (hydrogen and oxygen). Water is composed of two parts
hydrogen and one part oxygen. Hydrogen peroxide is composed of two parts hydrogen
and two parts oxygen. Hydrogen peroxide differs from water in that it has twice as
much oxygen. When you compare the mass of oxygen in hydrogen peroxide to the mass
of oxygen in water, you get the ratio 2:1.
PRACTICE
Problems
15.
A 78.0-g sample of an unknown compound contains 12.4 g of hydrogen. What is the percent by
mass of hydrogen in the compound?
16.
1.0 g of hydrogen reacts completely with 19.0 g of fluorine. What is the percent by mass of
hydrogen in the compound that is formed?
17.
If 3.5 g of element X reacts with 10.5 g of element Y to form the compound XY, what is the
percent by mass of element X in the compound? The percent by mass of element Y?
18.
Two unknown compounds are tested. Compound I contains 15.0 g of hydrogen and 120.0 g of
oxygen. Compound II contains 2.0 g of hydrogen and 32.0 g of oxygen. Are the compounds
the same? Explain your answer.
19.
CHALLENGE
All you know about two unknown compounds is that they have the same
percent by mass of carbon. With only this information, can you be sure the two compounds are
the same? Explain.
Get It?
State
the law of multiple proportions in your own words.
ADDITIONAL PRACTICE
Lesson 3 • Elements and Compounds
65









