
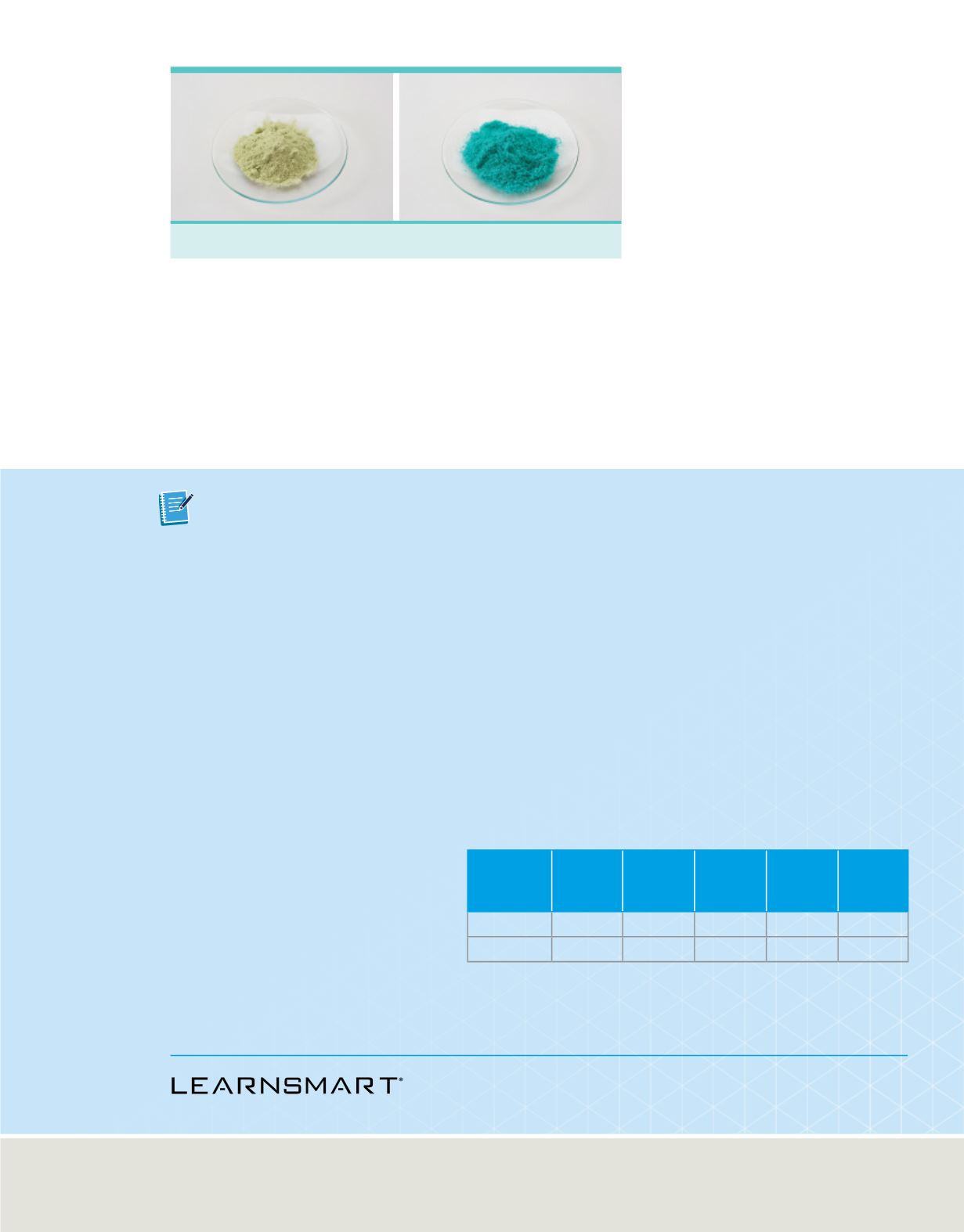
Figure 18
Different compounds are formed
when different relative masses of each
element are combined. Although they are
both made of copper and chlorine,
Compound I has a greenish color, whereas
Compound II has a bluish color.
Figure 18
shows the two compounds formed by the combination of copper and chlorine
and presented in
Table 4
and
Figure 17
. These compounds are called copper(I) chloride
and copper(II) chloride. As the law of multiple proportions states, the different masses of
copper that combine with a fixed mass of chlorine in the two different copper compounds
can be expressed as a small whole-number ratio. In this case, the ratio is 2:1. Considering
that there is a finite number of elements that exist today and an exponentially greater
number of compounds that are composed of these elements under various conditions, it
becomes clear how important the law of multiple proportions is in chemistry.
Compound I—copper(I) chloride
Compound II—copper(II) chloride
Go online to follow your personalized learning path to review, practice,
and reinforce your understanding.
Summary
• Elements cannot be broken
down into simpler substances.
• Elements are organized in the
periodic table of the elements.
• Compounds are chemical
combinations of two or more
elements, and their properties
differ from the properties of
their component elements.
• The law of definite proportions
states that a compound is
always composed of the same
elements in the same
proportions.
• The law of multiple proportions
states that if elements form
more than one compound,
those compounds will have
compositions that are
whole-number multiples of
each other.
Demonstrate Understanding
20.
Compare and contrast
elements and compounds.
21.
Describe
the basic organizational feature of the
periodic table of the elements.
22.
Explain
how the law of definite proportions applies to
compounds.
23.
State
the type of compounds that are compared in
the law of multiple proportions.
24.
Complete
the table. Then analyze the data to deter-
mine if Compounds I and II are the same compound. If
the compounds are different, use the law of multiple
proportions to show the relationship between them.
Check Your Progress
ANALYSIS DATA OF TWO IRON COMPOUNDS
Compound Total
Mass (g)
Mass
Fe (g)
Mass
O (g)
Mass
Percent
Fe
Mass
Percent
O
I
75.00 52.46 22.54
II
56.00 43.53 12.47
25.
Calculate
the mass percent of each element in water.
26.
Graph
Create a graph that illustrates the law of
multiple proportions.
Lesson 3 • Elements and Compounds
67
Matt Meadows/McGraw-Hill Education









